Question
Question: The structure of cis-bis(propenyl) ethene is: (a)  ethene is:
(a) 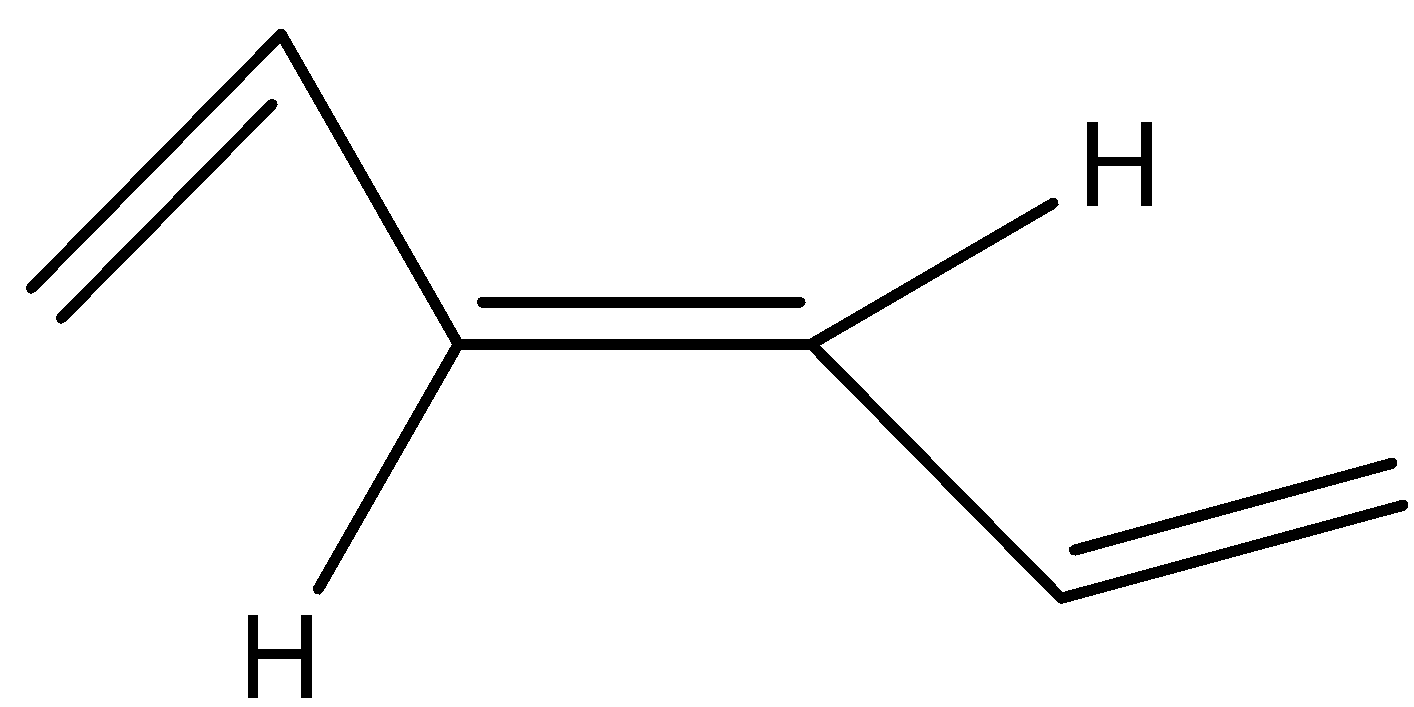
(b) 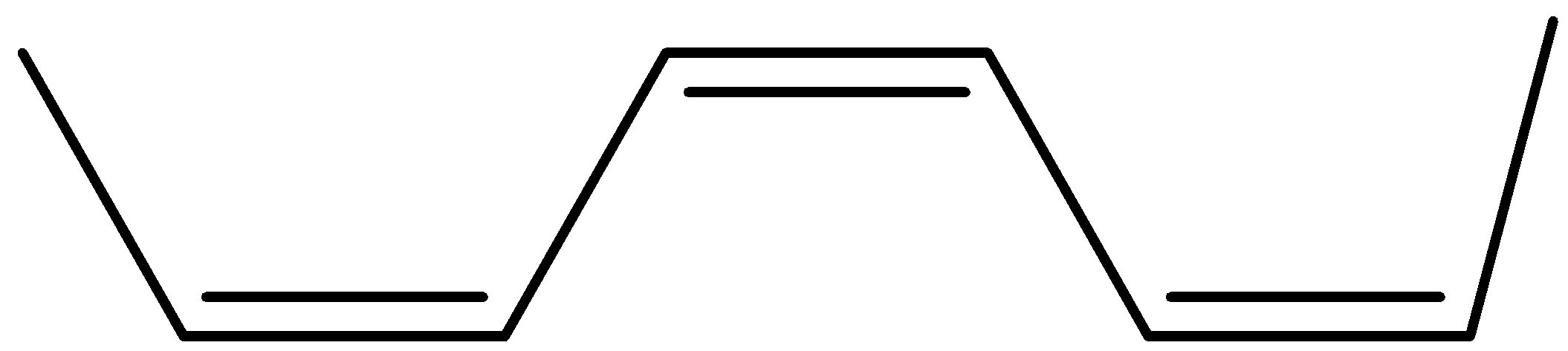
(c) 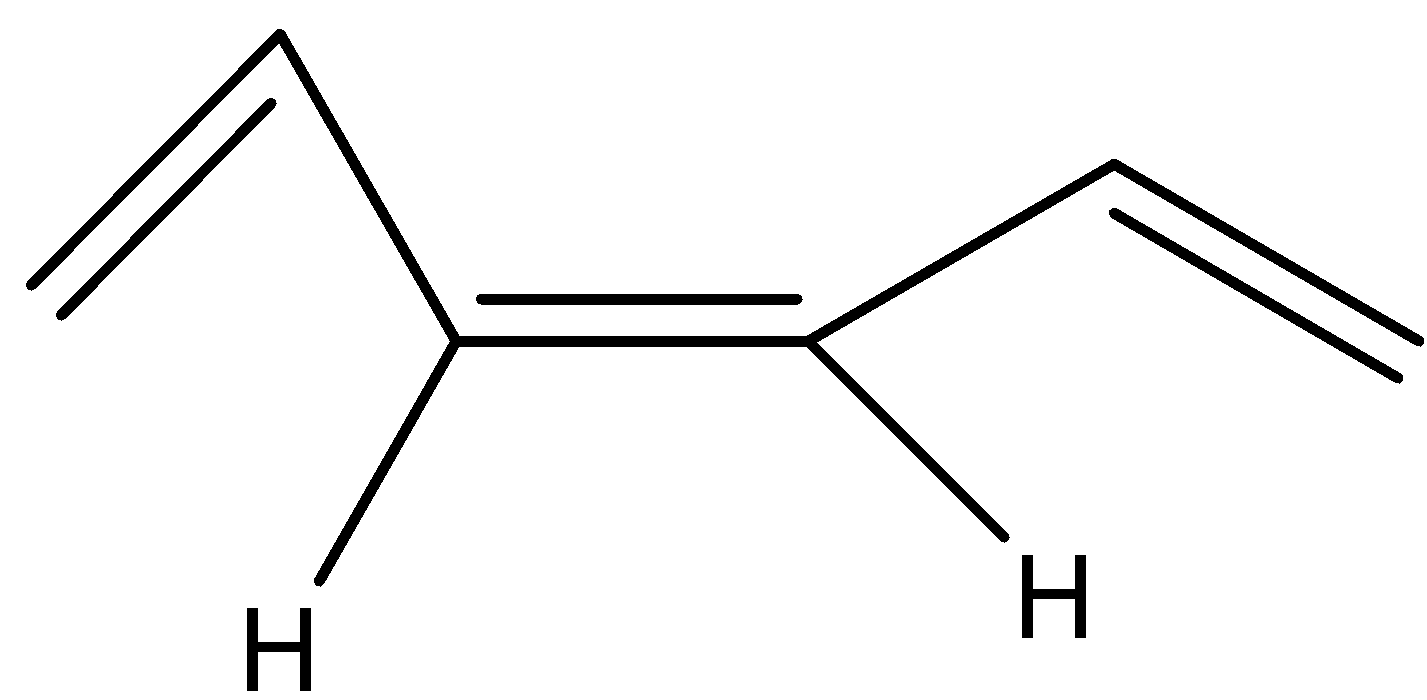
(d)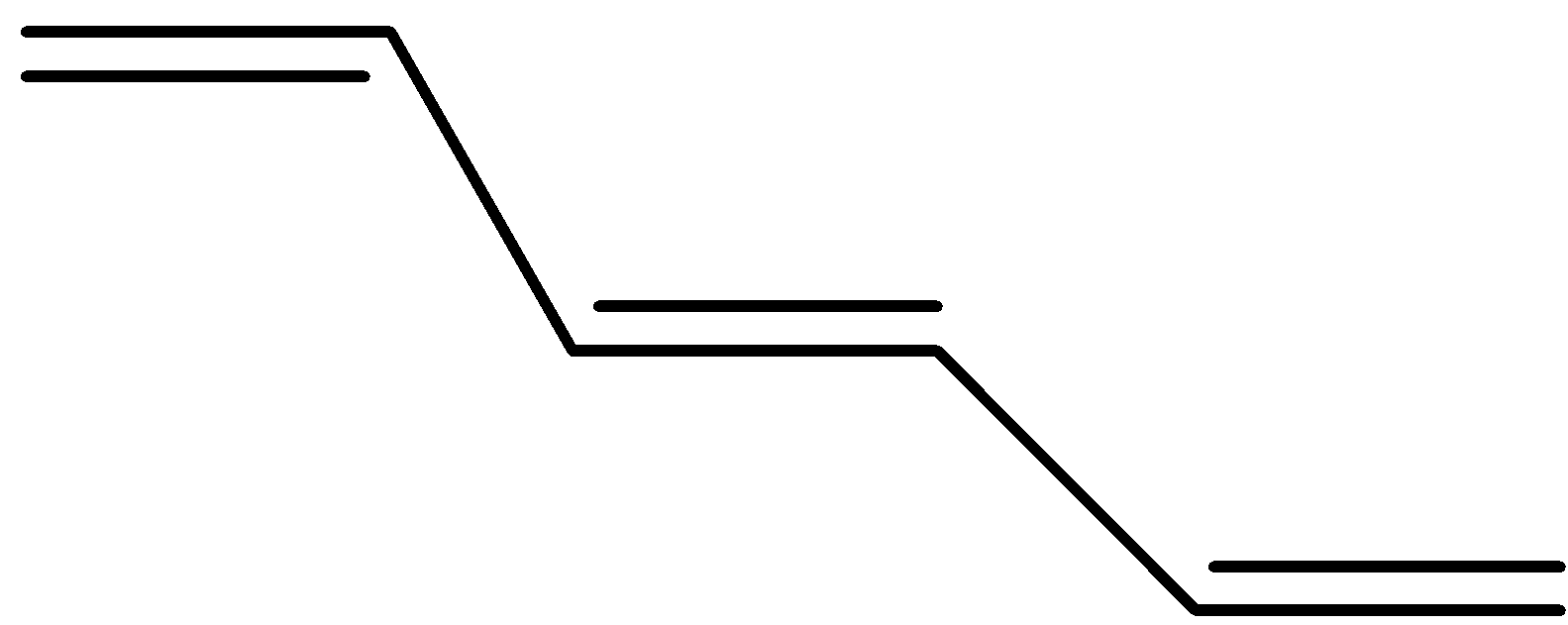
Solution
Cis isomer is where the same groups are on same side of an unsaturated bond. Trans isomer is when the same groups are on opposite sides of an unsaturated bond. This is only in the case of organic compounds.
Complete step by step answer:
The structure of bis(propenyl) ethene is
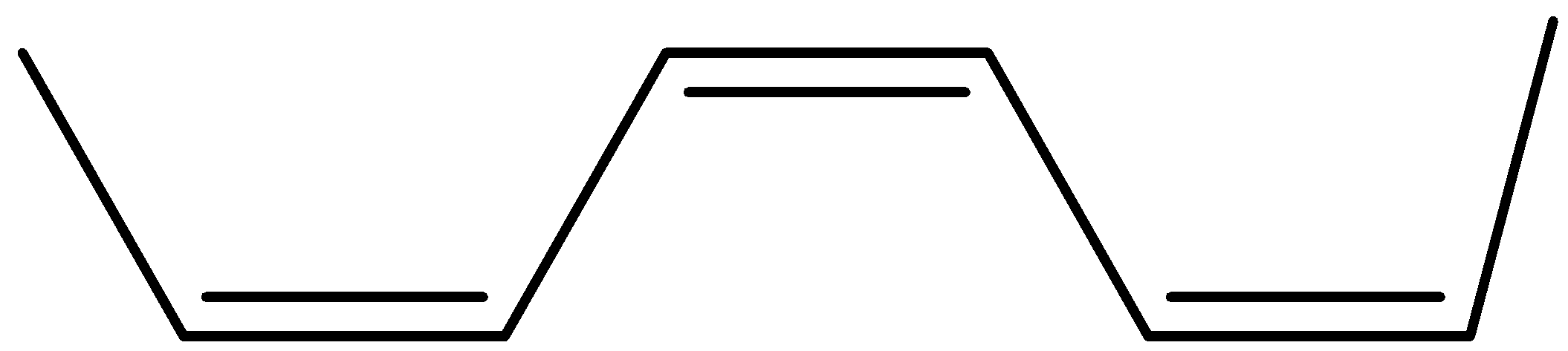
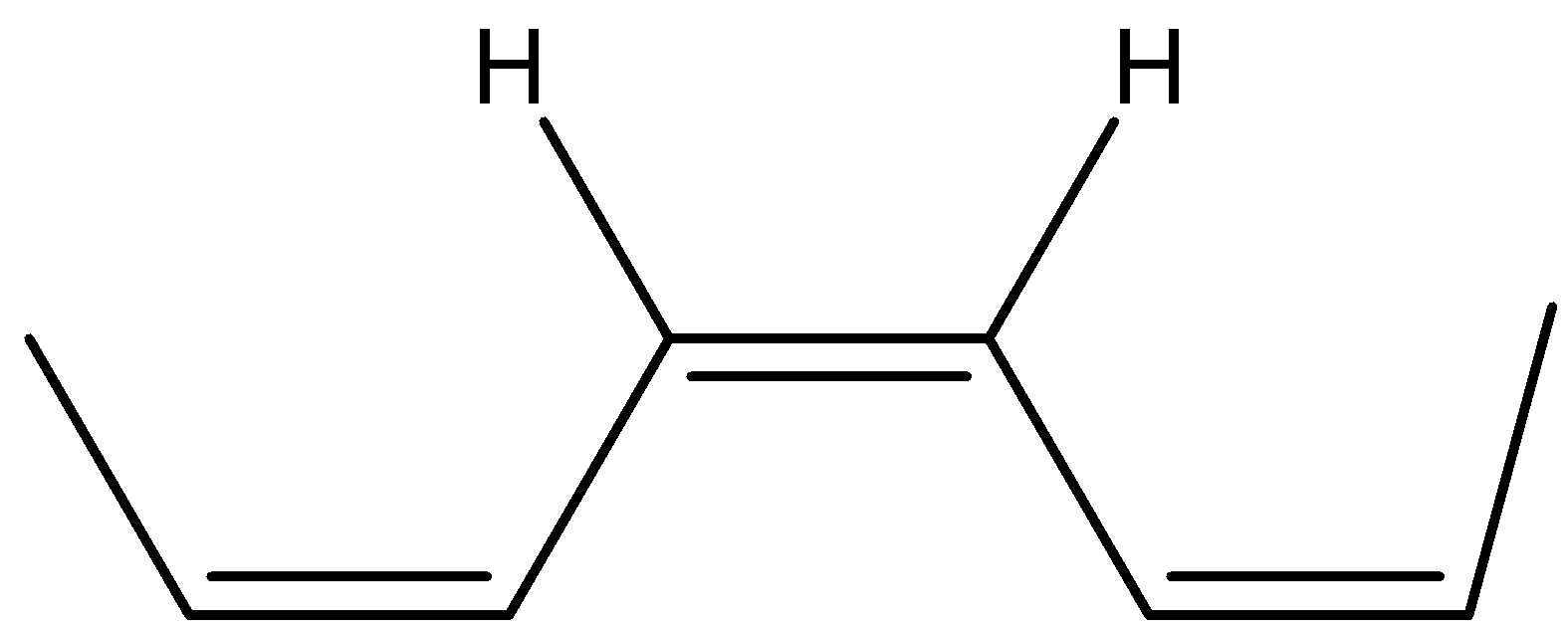
Now its cis isomer is where the 2 hydrogen will be on the same side and the alkyl groups on the other side.
The trans form the bis(propenyl) ethene will be where the hydrogen are in opposite sides or the alkyl groups are on opposite sides and its structure will be as shown below
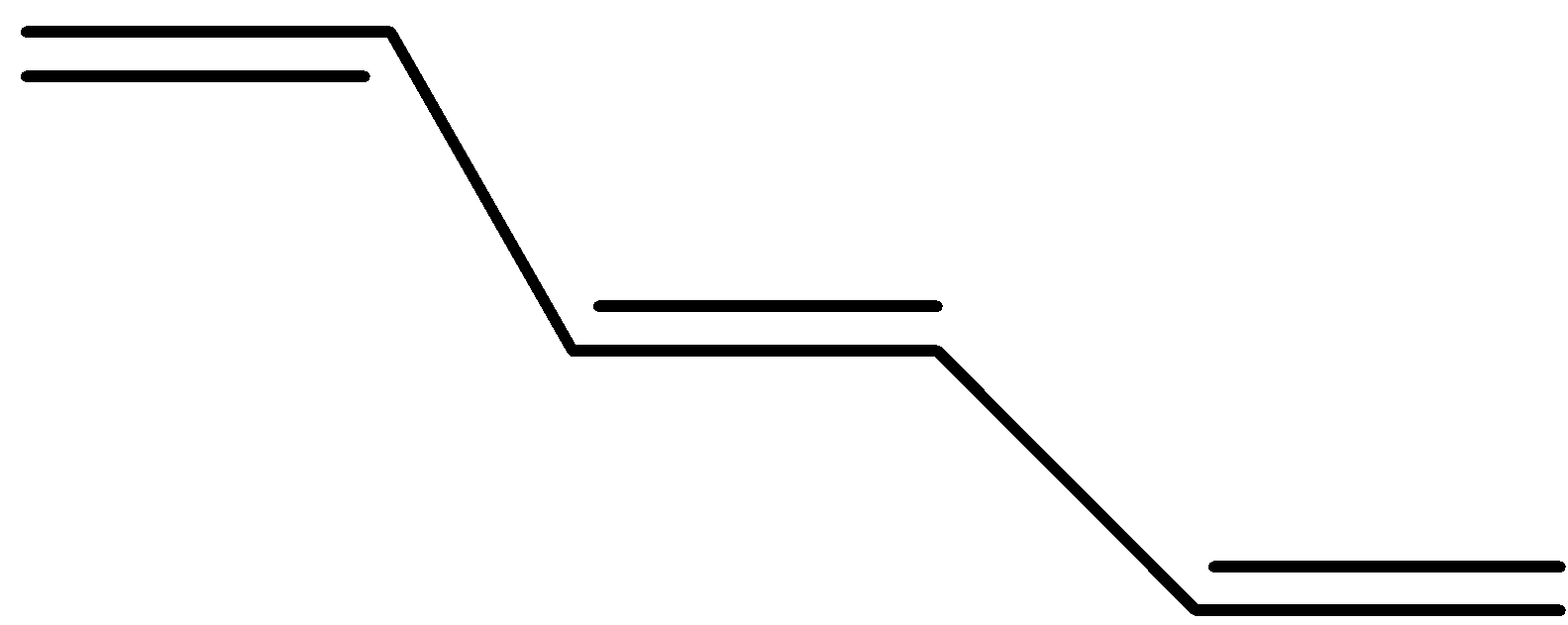
Hence, the correct answer to the question is option (b).
Additional Information:
Cis-Trans isomerism or the geometrical isomerism is also known as configurationally isomerism. Cis indicated that the functional groups are on the same side of the carbon chain while trans means that they are on opposing sides of the carbon chains. Cis and trans isomers are stereoisomer. These are pairs of molecules having the same formula but the functional groups are rotated into different orientations in three-dimensional space. This type of isomerism occurs in both organic and inorganic molecules. In inorganic molecules, it is seen in complexes.
Note:
Cis and trans isomers can only be formed if there is an unsaturated bond, that is, a double bond or a triple bond. It is an arrangement of the atoms around the double or a triple bond. Only in the case of organic compounds.
