Question
Question: The structure of \(C{{H}_{2}}=C{{H}_{2}}\) is (A)- Linear (B)- Planar (C)- Non-planar (D)- N...
The structure of CH2=CH2 is
(A)- Linear
(B)- Planar
(C)- Non-planar
(D)- None of the above
Solution
Hint : The carbon atoms in CH2=CH2 are sp2 hybridized. The hybridization of a molecule tells us about the geometry and shape of the molecule.
Complete step by step solution :
Let’s look at the answer
- The carbon atoms in the given molecule are both sp2 hybridized.
-The shape of sp2 hybridization is triangular planar.
-The carbon atom in the given molecule is linked to 2 sigma bonds to H atoms and one pi bond to another carbon atom.
-The pi bonds are formed by the lateral overlapping. For this the two atoms should be in the same plane for maximum overlap.
-In triangular planar geometry all the four atoms are in the same plane and the bond angles are120∘.
-The geometries of both the carbon atoms in the given molecule are triangular planar which is a planar geometry and the shape is also the same. Since, both the atoms are joined by double bonds so the molecule as a whole is planar.
Hence, the given molecule is a planar molecule.
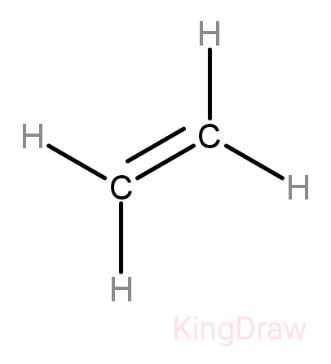
Structure of ethene
So, the correct answer is “Option B”.
Note : CH2=CH2is an alkene. In an alkene the single bond lengths and double bond lengths are different. It is also more acidic in nature than the corresponding alkane due to its geometry.
