Question
Question: The structural formula of mesityl oxide is:...
The structural formula of mesityl oxide is:
Solution
Hint The mesityl oxide is the compound that belongs to the α,β -unsaturated ketone means the carbon atom having the ketone group is the alpha-carbon and the beta-carbon will have the double bond. The molecular form of the mesityl oxide is C6H10O .
Complete step by step answer:
The mesityl oxide is the compound that belongs to the α,β -unsaturated ketone means the carbon atom having the ketone group is the alpha-carbon and the beta-carbon will have the double bond. The molecular formula of mesityl oxide is C6H10O and the IUPAC name of mesityl oxide is 4-methylpent-3-en-2-one. According to the name, the structural formula of the mesityl oxide will be:
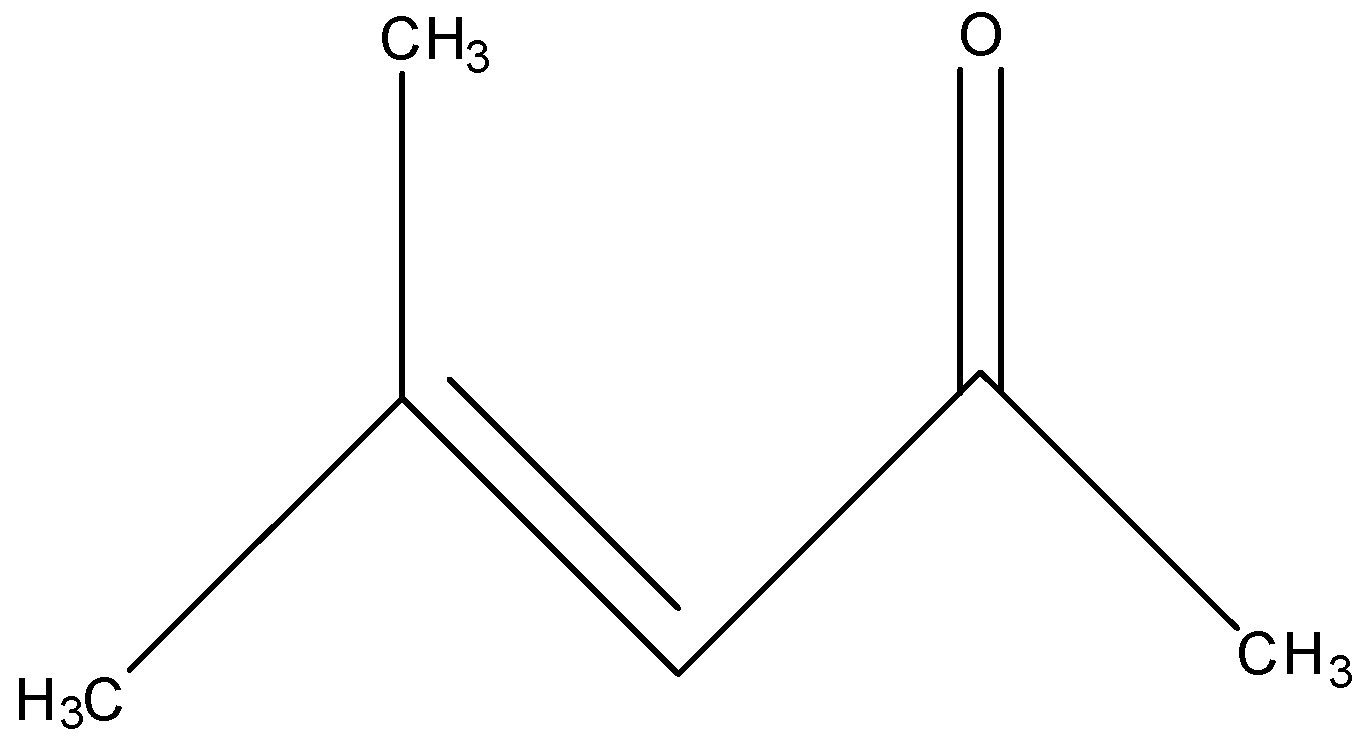
The other names of the mesityl oxide are isobutenyl methyl ketone, methyl isobutenyl ketone, and isopropylidene acetone.
The molecular mass of the mesityl oxide is 98.145 g/mol. The texture of the mesityl oxide is oily and its color ranges from colorless to light yellow, and it is a liquid.
It has a honey-like or peppermint like odor.
The density of the mesityl oxide is 0.858 g/cm3 .
The melting point of the mesityl oxide is 220 K or −53∘C and its boiling point are 402.6 K.
It is not soluble in water or its solubility is only 3% but in other solvents like organic solvents, it is soluble. The refractive index of mesityl oxide is 1.442. Its vapor pressure at 293 K is 9 mmHg. It is a flammable compound.
Note: It is mainly prepared by the aldol condensation of the acetone to give diacetone alcohol, which dehydrated to give mesityl oxide and the main use of mesityl oxide is as a solvent i.e., it is used as solvents in many reactions.
