Question
Question: The structural formula of hypophosphorous acid is: A.
B.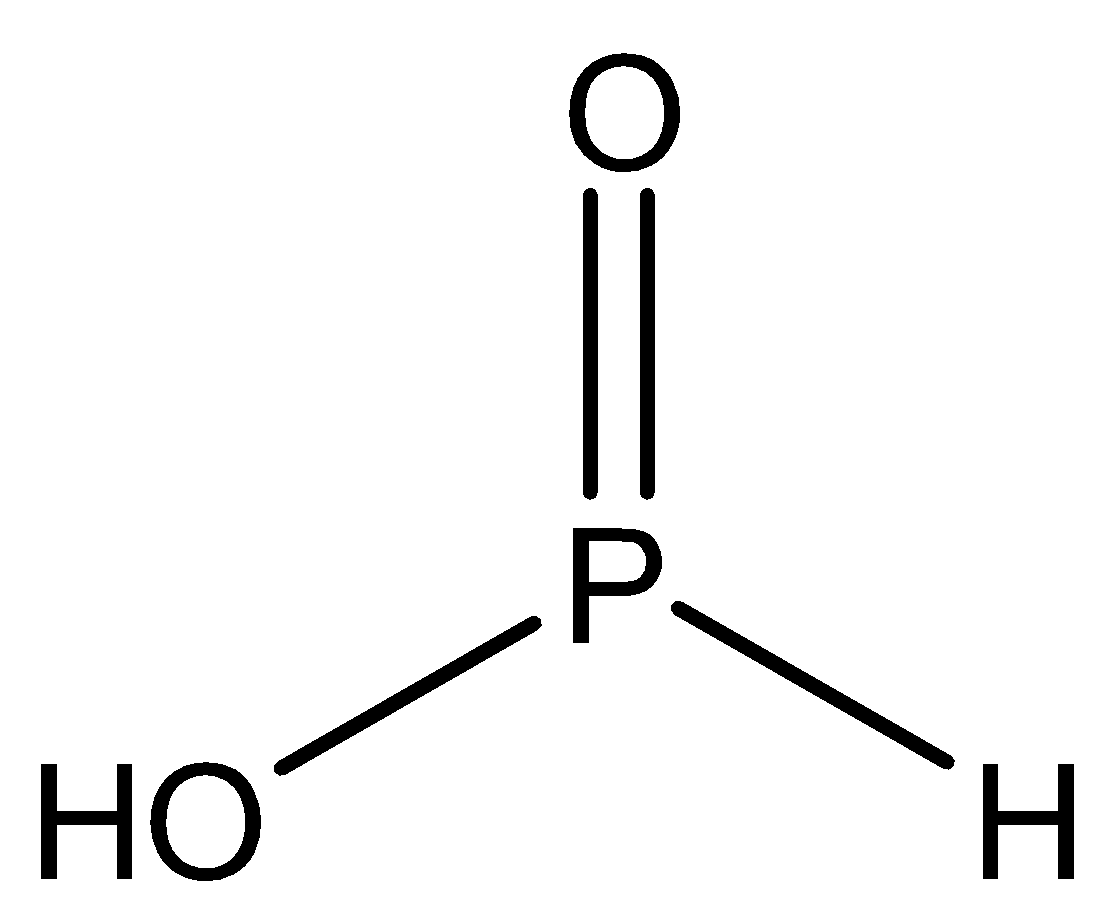
C.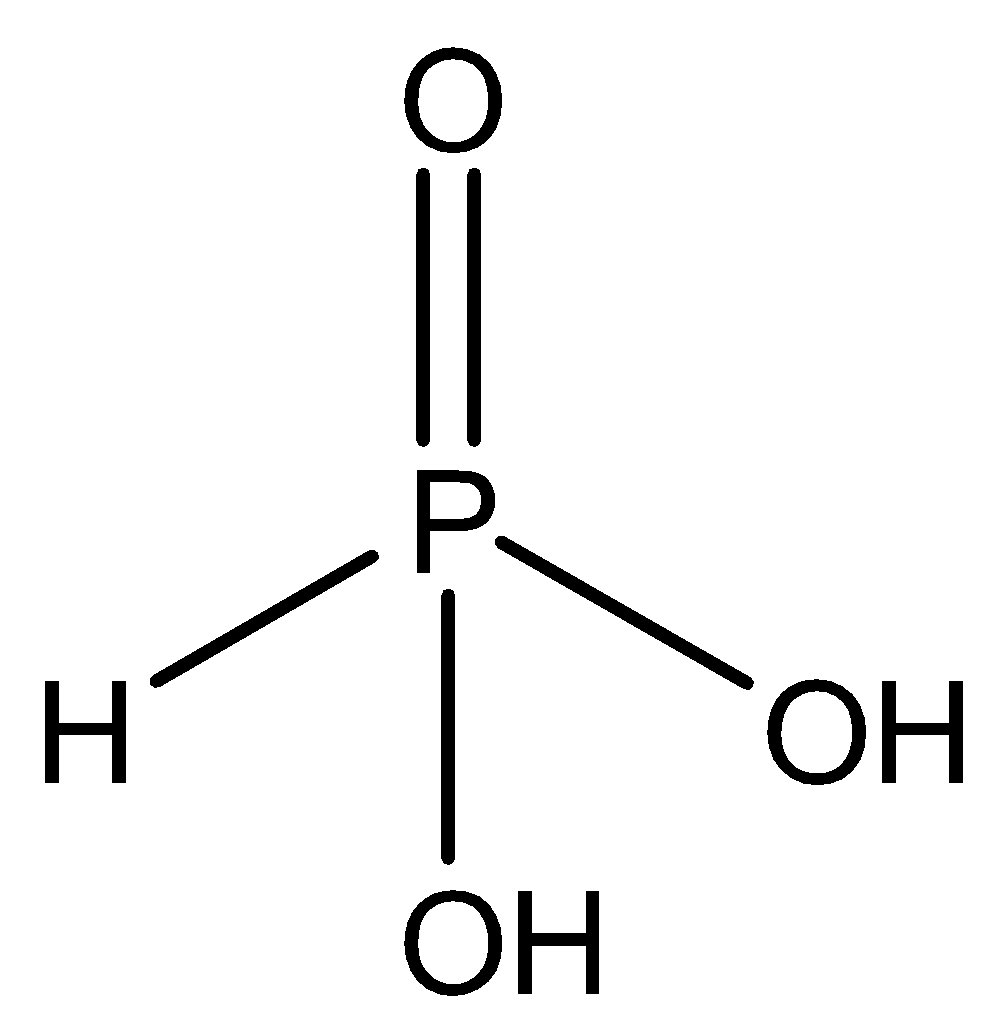
D.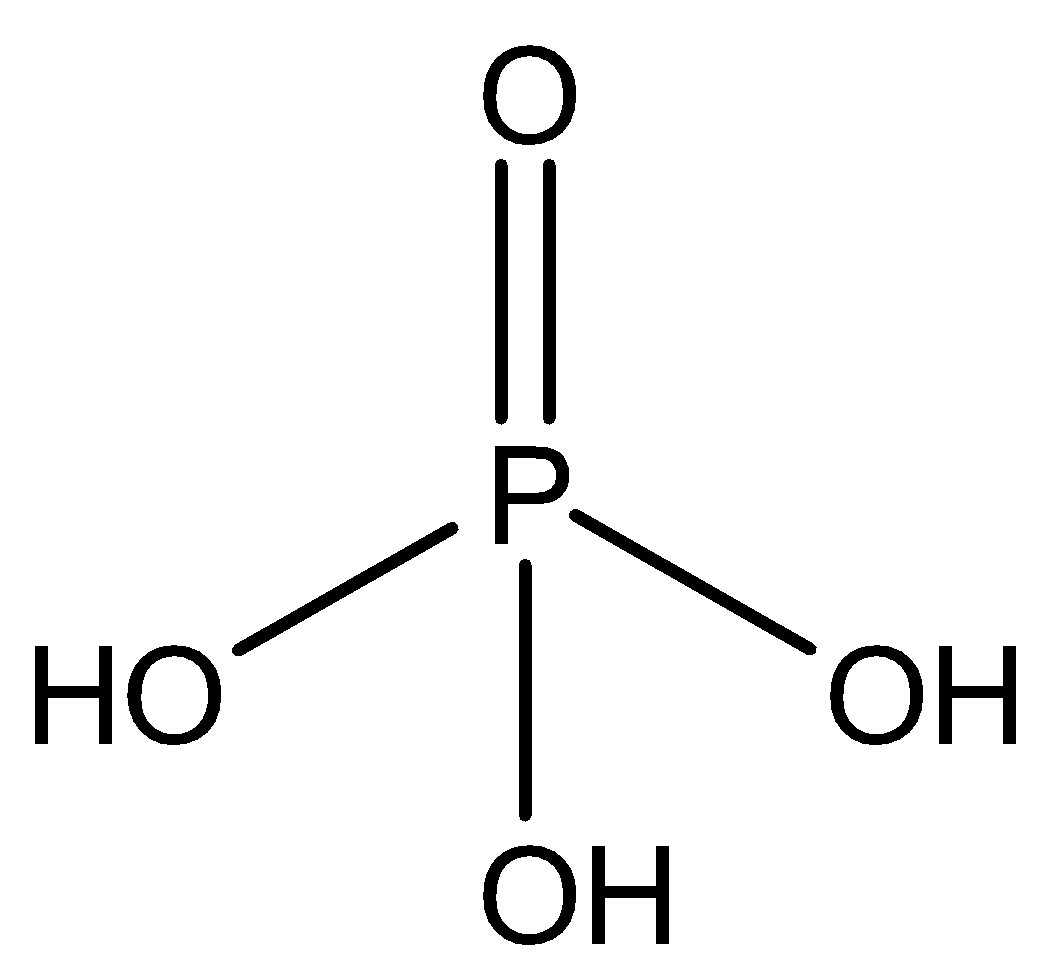
Solution
We need to remember that hypophosphorous acid is also called phosphinic acid. In chemistry, it is known to be a strong reducing agent. It is a phosphorus oxyacid which is physically colourless. It has a low-melting point and it is soluble in water, dioxane and alcohol.
Complete step by step answer:
We know that phosphorus has a valency of 5. IT means it can make 5 bonds with other atoms to satisfy its valency.
In the naming system, the polyatomic ions with two less oxygen atoms have the suffix “-ous” and prefix “hypo-”.
So according to the name hypophosphorous acid has two oxygen atoms less then the total number of oxygen atoms required.
Now, phosphorus atom will be linked with a double bond to one oxygen atom. And there are 3 more valencies to be satisfied. Thus, phosphorus will bond with two hydrogen atoms and one hydroxide atom. Thus, the structure will be,
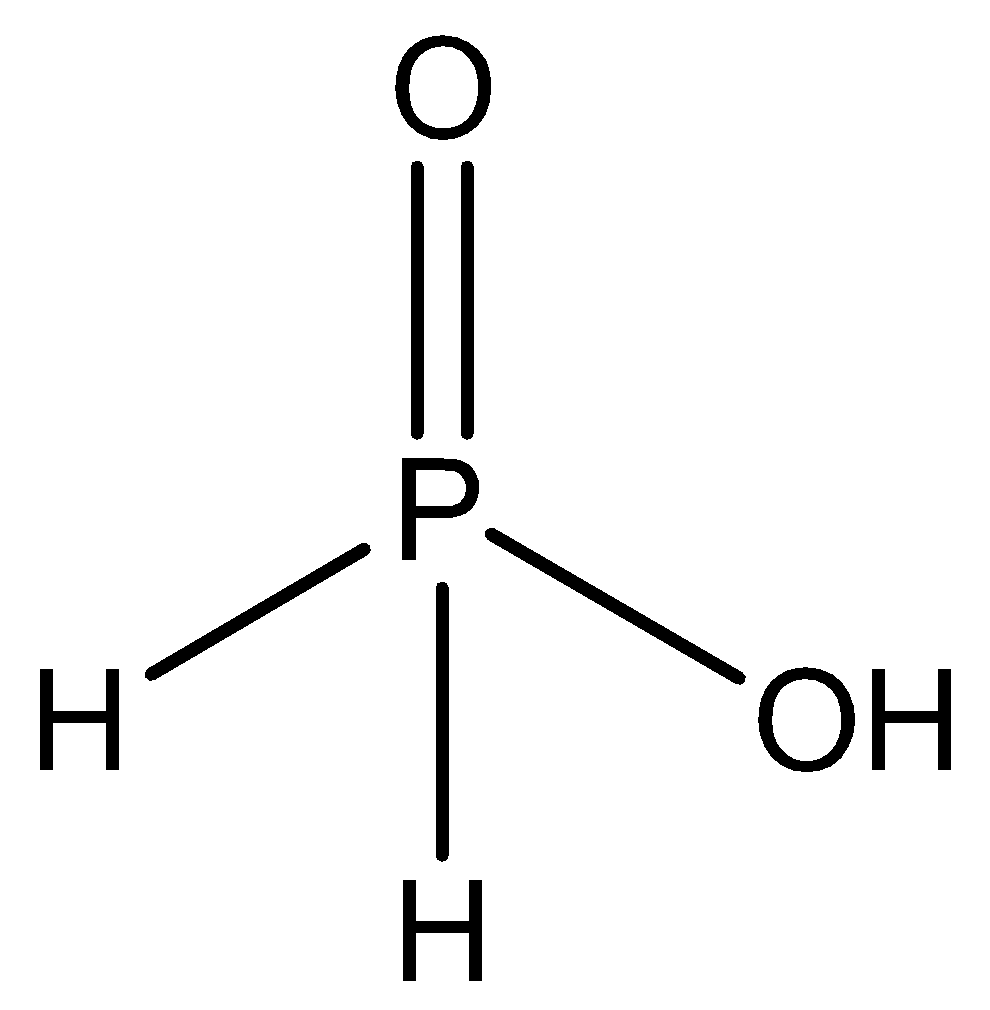
Thus, this structure is called as hypophosphorous acid.
The chemical formula is H3PO2. But descriptively it can also be written as - HOP(O)H2. Since, in a descriptive manner, we can clearly observe the bonding as well and determine the structure easily.
Hence, the correct answer is option A.
Note:
We should remember that hypophosphorous acid is monoprotic in nature. It implies it can donate protons and form a covalent bond. Sal derived from this acid are called hypophosphites. In industries, it is mainly used for electroless nickel plating. More ambiently it is used as a salt. In many organic chemical reactions, it is used as a reducing agent. It is used to reduce arenediazonium salts. It is used as an additive in fischer esterification reactions.
