Question
Question: The strongest acid amongst the following compounds is : (A)- \(C{{H}_{3}}COOH\) (B)- HCOOH (C...
The strongest acid amongst the following compounds is :
(A)- CH3COOH
(B)- HCOOH
(C)- CH3CH2CH(Cl)COOH
(D)- ClCH2CH2CH2COOH
Solution
Carboxylic acid is an organic compound having a carboxyl group attached to an alkyl or aryl group which reacts with metals and alkalis generating carboxylate ions. Such chemical reactions imply that carboxylic acids are acidic.
Complete Step by step solution:
-All the compounds given to us are carboxylic acid and its derivatives, so for identifying the strongest acid among all, we need to understand how the strength of acidity affects various substituents.
-Carboxylic acids in water dissociate forming carboxylate ion and the hydronium ion. The carboxylate anion formed will gain stabilization through resonance by effective delocalization of the negative charge.
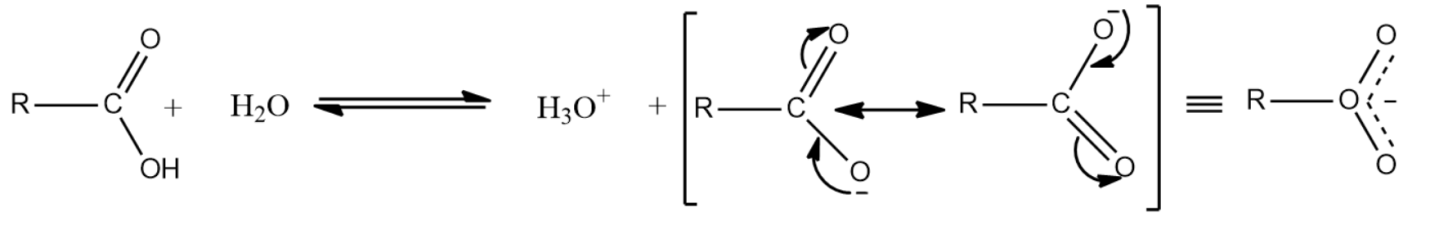
-The strength of the acid is determined by the stability of the conjugate base. Stable the conjugate base formed, stronger the acid.
-The acidity of carboxylic acid depends on the nature of substituent alkyl or aryl group attached to the carbonyl group.
-An electron-withdrawing group, when attached to the carbonyl group, ensures the effective delocalization of negative charge through renunciation of inductive effect which increases the stability of the conjugate base formed thus increasing the acidity of the carboxylic acids.
-An electron-donating group, when attached to the carbonyl group, destabilizes the conjugate base formed which decreases the acidity of carboxylic acids.
-Acidity of the carboxylic acid increases with the presence of a group with –I effect in the alkyl group and decreases with the presence of +I effect in the alkyl group.
-Acidity of carboxylic acids also increases with the increase in the number of halogen atoms on alpha position. As the distance of halogen from the carboxylic group increases, the acidity of the carboxylic acid decreases. Also, the increase in the electronegativity of the halogen increases the acidity.
-By comparing the nature of substituents of the carboxylic acids in the options given, we can determine the strongest acid among all these.
-On comparing the above-mentioned factors, the acidity will be highest in CH3CH2CH(Cl)COOH due to the presence of –I effect of chlorine atom on alpha carbon which increases the acidic strength significantly.
Therefore, the correct answer is option C.
Note: Carboxylic acids are of great significance in our day to day life. Carboxylic acid compounds and its derivatives are naturally occurring in different stages of the life cycle of living organisms such as Krebs cycle, fermentation processes and geological processes. Carboxylic acids can be produced in the laboratories or industries at large scale from the oxidation reactions of aldehydes, primary alcohol, and hydrocarbons, oxidative cleavage of olefins, base-catalyzed dehydrogenation of alcohols or through the hydrolysis of nitriles, esters or amides. Carboxylic acids and their derivatives are abundantly used in the production of polymers, biopolymers, coating, adhesives and pharmaceutical drugs.
