Question
Question: The strongest acid among the following is: (A) \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\t...
The strongest acid among the following is:
(A) C6H5CH2NH2
(B) C6H5COOH
(C) m - CH3OC6H4COOH
(D) p - CH3OC6H4COOH
Solution
Acidity can be said to be the tendency of a substance to give up a proton and basicity can be said to be the tendency of a substance to accept a proton. An acid is said to be strong if it has a greater tendency to donate or give up a proton. The relative strengths of acids and bases can be explained through inductive effects.
Complete step by step answer:
It has been found that the presence of electron withdrawing groups attached to the carboxyl group will increase the acidic strength whereas the presence of electron releasing groups attached to the carboxyl group will decrease the acidic strength.
On the other hand, the presence of electron donating or releasing groups increases the basic strength and the presence of electron withdrawing groups will decrease the basic strength.
Due to the presence of lone pair on the nitrogen atom, all amines are basic in nature. C6H5CH2NH2 is benzylamine which is an aralkylamine. In aralkylamines, the lone pair of electrons on the nitrogen atom is not conjugated with the benzene ring and hence not delocalized. So, they are more easily available for protonation and hence aralkylamines are very strong bases, even stronger than aniline. Thus, option A is not correct.
It is impossible to draw resonance structures involving the nitrogen and benzene ring because of the carbon between them.
C6H5COOH is benzoic acid and m - CH3OC6H4COOH and p - CH3OC6H4COOH are substituted benzoic acids called meta-anisic acid and para-anisic acid respectively.
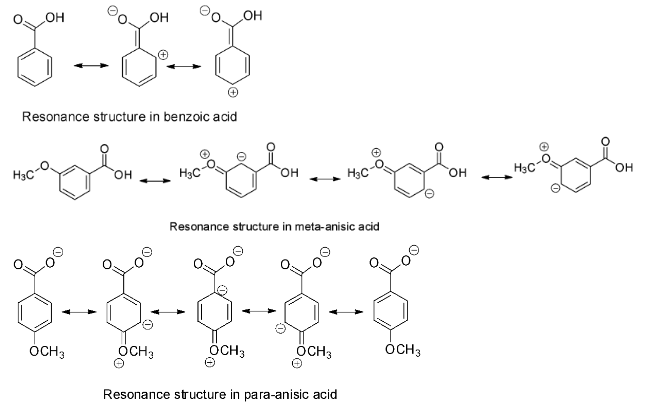
The acid weakening effect of the electron donating substituents and the acid strengthening effect of the electron withdrawing substituents is more pronounced at para position than at meta position. The OCH3 group has a strong +R resonance effect but a weak –I inductive effect. At para position, the OCH3 group shows its strong +R effect so para anisic acid is a weaker acid than benzoic acid. But at meta position, the OCH3 group can exert only its –I effect and so meta anisic acid is a stronger acid than benzoic acid. Therefore, the strongest acid among the given options is m - CH3OC6H4COOH .
So, option C is correct and the options B and D are wrong.
Note: The ortho substituted benzoic acids are usually stronger than benzoic acid regardless of the nature of the substituent. This is known as ortho effect. So, the ortho anisic acid should have been the strongest among all but due to stabilization by hydrogen bonding, the ortho effect is reduced and its acidic strength becomes equal to the meta isomer.
