Question
Question: The state of a gas in a cylinder is represented by the PV diagram shown below. The gas is taken thro...
The state of a gas in a cylinder is represented by the PV diagram shown below. The gas is taken through either the cycle ABCA or the reverse cycle ACBA. Which of the following statements about the work done on or by the gas is correct?
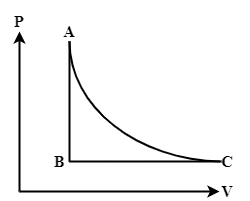
A) In both cases, the same amount of net work is done by the gas.
B) In both cases, the same amount of net work is done on the gas.
C) In cycle ABCA net work is done on the gas, in cycle ACBA the same amount of net work is done by the gas.
D) In cycle ABCA net work is done by the gas, in cycle ACBA the same amount of net work is done on the gas.
Solution
For any thermodynamic process, work done by a system is given by the area under the process presented in the PV diagram. Hence, the sum of individual work done in each of the three steps would give total work done by the gas in the cyclic process.
Complete step by step solution:
Here, the area under the curve gives work done by the gas for that particular process. Now, the value of this area follows a convention depending on which direction the process is happening in the curve. If the process happens in such a way that volume increases then work done by the system is positive. Hence, the area is also taken as positive. In the opposite direction of the process, the area is considered negative.
Step 1:
Hence, calculate work through the ABCA cycle as:
For path AB (from A to B) total work done is zero, i.e. WAB=0.
For path BC (from B to C) total work done is WBCand this work is positive.
For path CA (from C to A) total work done is −WCAand this work is negative.
From the graph it is evident that the area under the AC curve is greater than the BC curve. So, work done by the gas in cycle ABCA is:
Step 2:
Now, calculate work through the ACBA cycle as:
For path AC (from A to C) total work done is WACand this work is positive.
For path CB (from C to B) total work done is −WCBand this work is negative.
For path BA (from B to A) total work done is zero, i.e. WBA=0.
So, work done by the gas in cycle ABCA is:
Hence, you get the relation as: WACBA=−WABCA.
The correct statement is in option (C). In cycle ABCA, the net work is done on the gas, in cycle ACBA the same amount of net work is done by the gas.
Note:
Although the total area of the encircled region is the same for both the cyclic process you must always remember that the nature of it changes as the direction of the cyclic process changes. For two opposite cycles work done is the opposite. One is on the gas, the other is by the gas.
