Question
Question: The ‘spin only’ magnetic moment of \(N{i^{2 + }}\) in aqueous solution would be: A. \(\sqrt 6 BM\)...
The ‘spin only’ magnetic moment of Ni2+ in aqueous solution would be:
A. 6BM
B. 15BM
C. 2BM
D. 8BM
Solution
The net magnetic moment of an atom/ion is the vector sum of its orbital and spin magnetic moments. We can easily calculate the spin only magnetic moment using the formula uspin=n(n+2) where n is the number of unpaired electrons present in an atom or ion. Bohr magneton or BM is the unit of magnetic moment.
Complete step by step answer:
Ni is present in Ni2+ oxidation state as [Ni(H2O)6]2+
First of all, we know that Ni is a d-block element and H2O being an electron rich species (due to presence of two lone pairs on oxygen atoms) can easily form a coordinate compound.
We look, into the electronic configuration of Ni2+ ion [Ni(H2O)6]2+
Ni=1622s22p63s23p63d84s0
Ni2+=1s22s22p63s23p63d84s0
Thus, according to valence and theory (VB+) water being a ligand form of a high spin complex with Ni2+ and this can be as show below
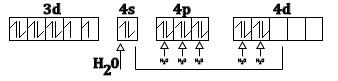
The electrons are filled according to Hund’s rule. Thus, the spin only magnetic moment can be easily calculated using the data given above. As we have seen there are two unpaired electrons in the complex compound so
uspin=n(n+2)BM
=2(2+2)BM
=8BM
Where, BM= Bohr magneton
Hence, option (d) is the correct option.
Note:
Student can also use an alternative method to calculate the spin only magnetic moment of the atom or ion by using a formula
uspin=4s(s+1)BM
Where, s= angular spin moment quantum number.
For Ni2+ with two unpaired electrons
s=21+21=1
Therefore, uspin=4×1(1+1)BM
⇒8BM which is the required solution.
