Question
Question: The species in which the N atom is in a state of sp hybridization is: \[ {A:{\text{ }}N{O_2}^ ...
The species in which the N atom is in a state of sp hybridization is:
A: NO2− B: NO3− C: NO2 D: NO2+Solution
Orbital hybridisation or simply hybridization is the concept in chemistry which states that mixing of the atomic orbitals into the new hybrid orbitals (which possess different shapes, energies, etc., in comparison to component atomic orbitals) is suitable for the electron pairing in order to form chemical bonds in the valence bond theory. Hybridization is generally used to explain molecular geometry of organic compounds.
Complete step by step answer
To find out the hybridization around the N atom, you will have to carry out the summation of the number of sigma bonds as well as the number of pairs of lone electrons around the N atom. Let us check the hybridization around N atom in each case:
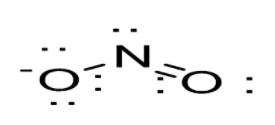
Option A: NO2−leads to the formation of two bonds. N atoms possess a total of six outer electrons out of which two form a lone pair of electrons. Thus, one s and two p orbitals are being hybridized so we get sp2 hybridization and shape obtained is trigonal-planar as shown below:
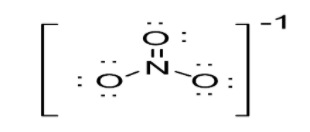
Option B: NO3−leads to the formation of three bonds as N atom is bonded to three O atoms. Thus, N atom is left with no lone pair of electrons. N atoms possess a sp2 hybridization. As a result in case of NO3−, trigonal planar geometry exists as shown below:
Option C: NO2leads to the formation of two bonds. N atoms possess a sp2 hybridization. As a result in case of NO2, trigonal planar geometry exists as shown below:
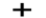
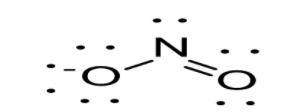
Option D: NO2+leads to the formation of two bonds. The molecule possesses four outer electrons out of which two form sigma bonds and two form pi bonds. Thus, one s and one p orbital are being hybridized resulting in sp hybridization. As a result in case of NO2+, linear geometry exists as shown below:
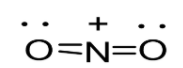
Note:
The sequence of hybrids according to energy level is sp < sp2 < sp3. If p character is higher, it means that the energy is more, thus indicating that the electrophilicity is higher and moreover, its affinity for reaction is also higher. It should also be noted that hybrids (sp, sp2, sp3) lead to the formation of σ bonds and pure-breeds on the other hand, lead to the formation of π bonds.
