Question
Question: The source of nitrogen in Gabriel synthesis of amines is: A. Sodium azide \(Na{N_3}\) B. Sodium...
The source of nitrogen in Gabriel synthesis of amines is:
A. Sodium azide NaN3
B. Sodium nitrite NaNO2
C. Potassium cyanide KCN
D. Potassium phthalimide C6H4(CO2)N−K+
Solution
the Gabriel synthesis is a method used to prepare primary amines.
Complete step by step solution: while preparing amines when ammonia is reacted with alkyl halides it results in multiple alkylations which can be reduced by using a large excess of ammonia. Hence phthalimide can be used. It is quite acidic in nature so it is converted to potassium phthalide by reacting it with potassium hydroxide.
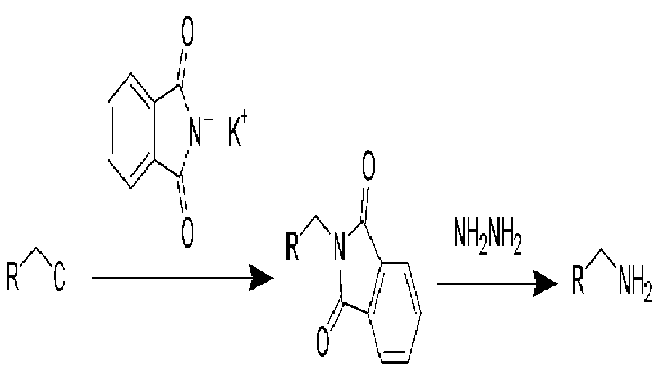
The phthalimide anion is a strong nucleophile and it reacts with an alkyl halide by a nucleophilic reaction of SN2 to give N-alkyl phthalimide. At this point the N-alkyl phthalimide can be hydrolyzed by with aqueous acid or base but the hydrolysis is often difficult it is often more convenient to treat the N-alkyl phthalimide with hydrazine () in refluxing ethanol to give a primary amine and phthalazine-1, 4-dione. Synthesis of amines using the Gabriel synthesis is, as we might expect restricted to the use of methyl, primary and secondary alkyl halides. The use of tertiary halides leads almost exclusively to eliminations.
Hence the correct option is option D as potassium phthalimide is used in Gabriel synthesis.
Additional information: Multiple alkylations are not a problem when tertiary amines are alkylated with methyl or primary amines. Such reactions take place in good yield
Note: This method is used to avoid multiple alkylations that occur while treating amines with alkyl halides.
