Question
Question: The solubility of $Ca(CN)_2$ in 0.05 M NaCN solution is $x \times 10^{-6}$. The $K_{sp}$ of $Ca(CN)_...
The solubility of Ca(CN)2 in 0.05 M NaCN solution is x×10−6. The Ksp of Ca(CN)2 is 1.0×10−8. Find the value x.
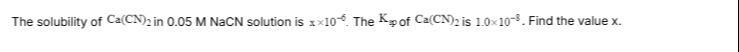
Answer
x = 4
Explanation
Solution
For the dissolution of Ca(CN)2:
Ca(CN)2(s)→Ca2+(aq)+2CN−(aq)Let the solubility of Ca(CN)2 be s mol/L. Then:
[Ca2+]=sand[CN−]=0.05+2s≈0.05(since 2s≪0.05)The solubility product is:
Ksp=[Ca2+][CN−]2≈s×(0.05)2 1.0×10−8=s×0.0025 s=0.00251.0×10−8=4.0×10−6Thus, x=4.
In summary:
Assume solubility s. [CN−] is approximately 0.05 M. Set up Ksp=s(0.05)2. Solve for s to find s=4.0×10−6, so x=4.
