Question
Question: The solubility curve of \(KN{O_3}\) in water is shown below. The amount of \(KN{O_3}\) that dissolve...
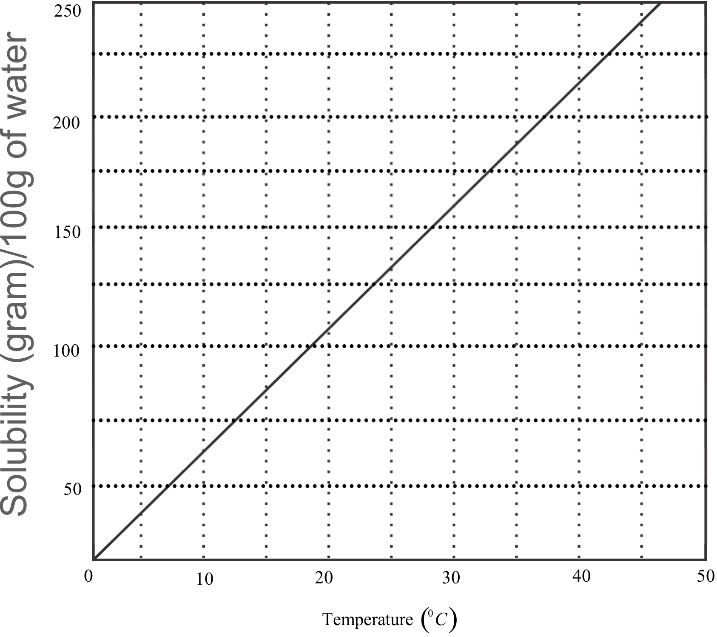
The solubility curve of KNO3 in water is shown below. The amount of KNO3 that dissolves in 50g of water at 40∘C is closest to
A. 100g
B. 150g
C. 200g
D. 50g
Solution
As we need to find out the solubility at 40∘C, we can draw a line from the point 40∘C in the x−axis to meet the curve. From this meeting point, we can draw a horizontal line to the y−axis to find the solubility per 100g. Dividing this value by two will give us our answer.
Complete step by step answer:
Since the temperature is given in the x−axis, we have to first draw a vertical line from the point 40∘C and extend it upwards till it meets the solubility curve. From the point of intersection of this line and the solubility curve, we should draw a horizontal line and extend it till it meets the y−axis. The point of intersection of this horizontal line and the y−axis will give us the value on the y−axis corresponding to the value on the x−axis. Since the intersection point is closest to 200g, we can say that at a temperature of 40∘C, the solubility of potassium nitrate (KNO3) per 100g of water is 200g. But we are required to find the quantity that dissolves in 50g of water at 40∘C , and not 100g. Therefore, to get the answer per 50g, we just have to divide the answer which we got per 100g by two. Hence,
Amount of potassium nitrate that dissolves in 50g of water at 40∘C =2200=100g
Hence, the correct option to be marked is option A.
Note:
Solubility of a salt in water is dependent on lattice enthalpy (energy required to break the intermolecular forces of attraction between the salt molecules) and solvation enthalpy (energy released when the salt is dissolved in water). Thus, for a salt to be soluble in a solvent, its solvation enthalpy must be higher than its lattice enthalpy. Also note that as temperature increases, the solubility of most salts in a particular solvent tends to increase.
