Question
Question: The solubility curve of \( KN{{O}_{3}} \) in water is given below. The amount of \( KN{{O}_{3}} \) t...
The solubility curve of KNO3 in water is given below. The amount of KNO3 that dissolves in 50g of water at 400C is closest to:
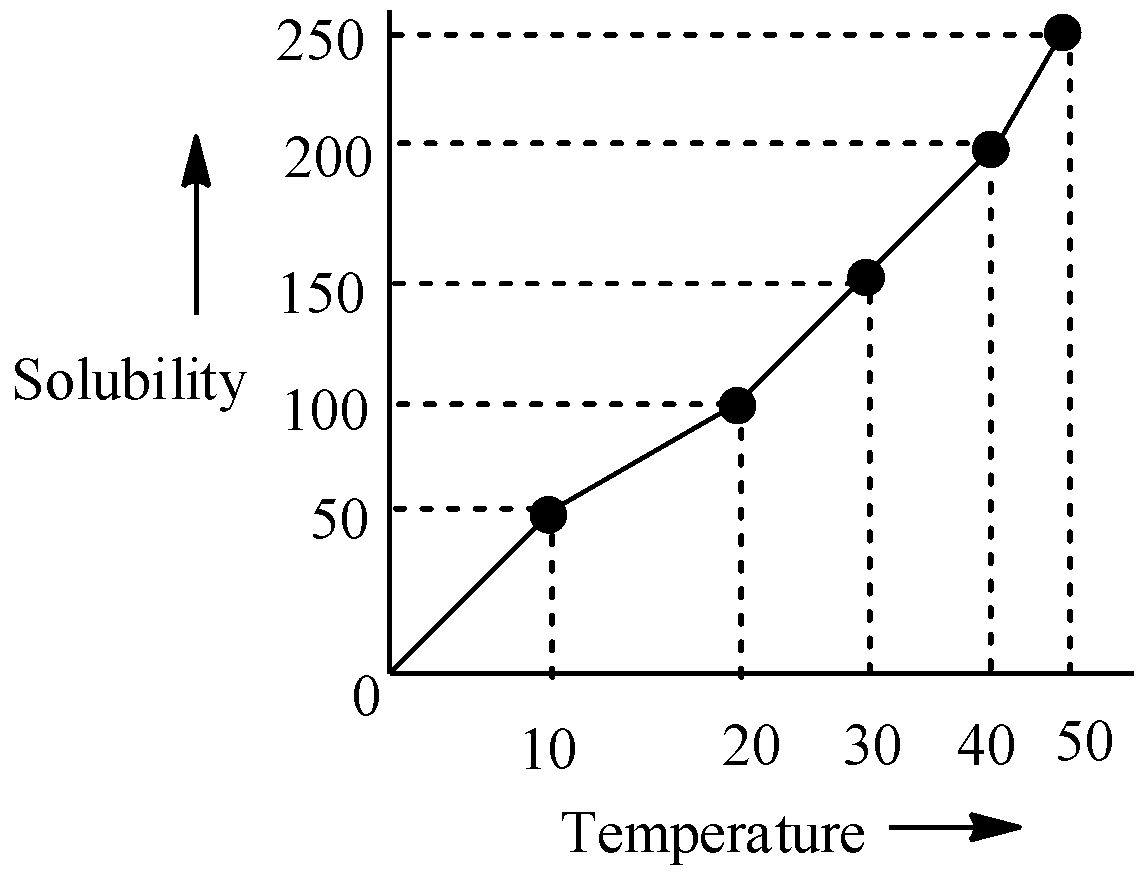
A.100g
B. 150g
C. 200g
D. 50g
Solution
Hint Choose any specific point on the graph, preferably at 400C and observe the solubility at that region. Use a unitary method to derive to a final conclusion.
Complete step by step solution:
In order to answer the question, we need to learn about solute, solvent and solutions. Now, in a mixture of two substances, one substance is present in low quantity and the other substance is present in more quantity. So, the substance that is present in smaller quantities is called the solute and the substance that is present in more quantities is called the solvent. In a binary mixture, both the solute and the solvent together is known as a solution.
Now, the solubility of the solute in a particular solvent depends upon many factors, one of them being the temperature. It is observed that by increasing the temperature, the solubility increases. For example, we can keep adding salt in 100mL of water but at one time no more salt will get dissolved in it. However, if we heat the water now, we can dissolve more salt in it. Now, if we cool down the water back to its normal temperature then the extra salt that was dissolved, will crystallize out. This is how temperature plays a role in the solubility.
Let us come to the question now. From the graph we can infer that as the temperature is increasing, the solubility is increasing too and it is more or less a straight line curve. We will pick up a specific point on the graph which is at 400C . At this temperature, 200g of KNO3 can get dissolved in 100g of water. So, if we multiply the mass of water by 21 , then we can infer that 50g of water will dissolve (21×200)g of KNO3 , which is 100 grams, at 400C .
So, we obtain the correct answer as option A.
NOTE: The reason for the increased solubility, on increasing the temperature accounts for the fact that as heat is gained by the solid the kinetic energy increases and facilitates the breaking of bonds that are held together by intermolecular attractions.
