Question
Question: The smallest ketone and its net homologue are reacted with \[N{H_2}OH\] to form oxime- A.Two diffe...
The smallest ketone and its net homologue are reacted with NH2OH to form oxime-
A.Two different oximes are formed
B.Three different oximes are formed
C.Two oximes are optically active
D.All oximes are optically active
Solution
Ketones are the carbonyl compounds that react with hydroxyl amine and undergo a loss of water molecule to form an oxime. The smallest ketone is acetone and the next homologue is butanone. These both ketones reacted to form two oximes and were formed as optically active compounds.
Complete answer:
Organic compounds are of different types. Ketones and aldehydes belong to carbonyl compounds. Ketones consist of a carbonyl group attached to two different carbons or two alkyl groups. The smallest ketone is acetone it has the molecular formula of C3H6O The next homologue is butanone it has the molecular formula of C4H8O .
When acetone is treated with hydroxylamine, acetoxime will be formed by the elimination of water molecules.
CH3COCH3+NH2OH→CH3C(NOH)CH3
The product is acetoxime. It is an optically active oxime.
When butanone reacts with hydroxylamine, an oxime will be formed by the elimination of water molecules.
CH3COC2H5+NH2OH→CH3C(NOH)C2H5
The chemical reaction of the two ketones will be as follows:
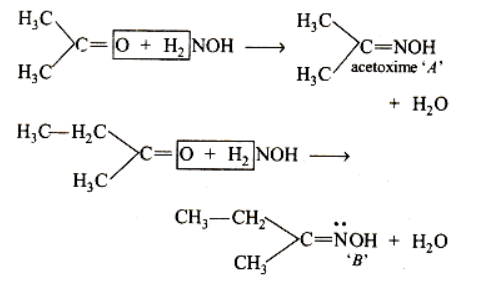
The two oximes contain a different group and do not have a plane of symmetry. Thus, these two oxides are optically active compounds.
Option B is the correct one.
Note:
The above oxime B has lone pair of electrons on nitrogen atom. This lone pair of electrons leads to the formation of two geometrical isomers. Geometrical isomers have a different stereo arrangement around the carbon atom, oxime B has two different stereo arrangements.
