Question
Chemistry Question on The Valence Shell Electron Pair Repulsion (VSEPR) Theory
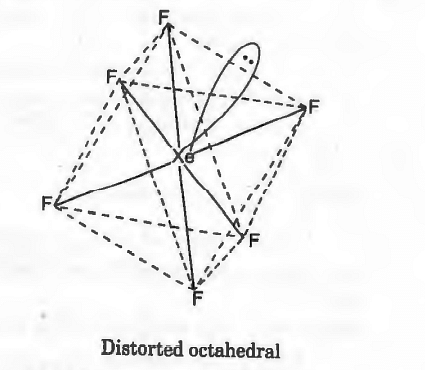
The shape of XeF6 is,
A
Square planar
B
Distorted octahedral
C
Square pyrimidal
D
Pyramidal
Answer
Distorted octahedral
Explanation
Solution
In XeF6, there are 6σ -bonds and one lone pair of electrons. Thus, it has distorted octahedral structure