Question
Chemistry Question on p -Block Elements
The shape of XeF5− will be
A
Square pyramid
B
Trigonal bipyramidal
C
Planar
D
Pentagonal bipyramid
Answer
Planar
Explanation
Solution
Shape of XeF5−→
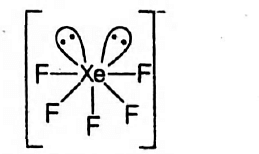
The Xe show sp3d3-hybridisation in XeF5−,
Thus, its geometry is Pentagonal-bipyramidal.
But, number of electron pair =28+5+1=7
Number of bond pair =52, number of lone pair =2 .
A lot of electrons are present at axil position and all bonds are in same plane.
Hence, the shape is planar.
