Question
Question: The shape of \[Xe{{F}_{4}}\] is: - (a)- tetrahedral (b)- octahedral (c)- square planar (d)-...
The shape of XeF4 is: -
(a)- tetrahedral
(b)- octahedral
(c)- square planar
(d)- pyramidal
Solution
The shape of XeF4 is based on the number of atoms joined to the central atom. Not only atoms, but lone pairs are also considered for the shape. For finding the shape of a compound the number of total number of electron pairs and number of lone pairs should be calculated with the help of valence electrons, number of bonds etc.
Complete step by step answer:
Both the VSEPR theory and the concept of hybridization are applied to predict the molecular geometries of xenon compounds.
According to the VSEPR theory, the shape of the molecule is predicted by the total number of electron pairs (lone pairs + bond pairs) in the valence shell of the central Xe atom.
To calculate the total number of electron pairs:
2valence electrons of central atom + number of bonded atoms
With the above formula: 28+4=6
Hence, there are 6 electron pairs.
Since there are 4 fluorine atoms joined to xenon. So, there will be a 4bond pair of electrons.
Now for calculating the number of lone pairs in the compound: -
total number of electron pairs –number of bond pairs.
Lone pairs=6−4=2.
Hence, in the compound, there are 2 lone pairs.
Depending on the number of Xe−F covalent bonds to be formed, the requisite number of electrons of the of the5p−orbital valence shell of Xe get unpaired and promoted to the vacant 5d−orbitals followed by hybridization.
Since, there are 6 electron pairs, the hybridization of the compound will be sp3d2.
So, the hybridization is sp3d2 and it has 2 lone pairs, the shape of XeF4 is square planar.
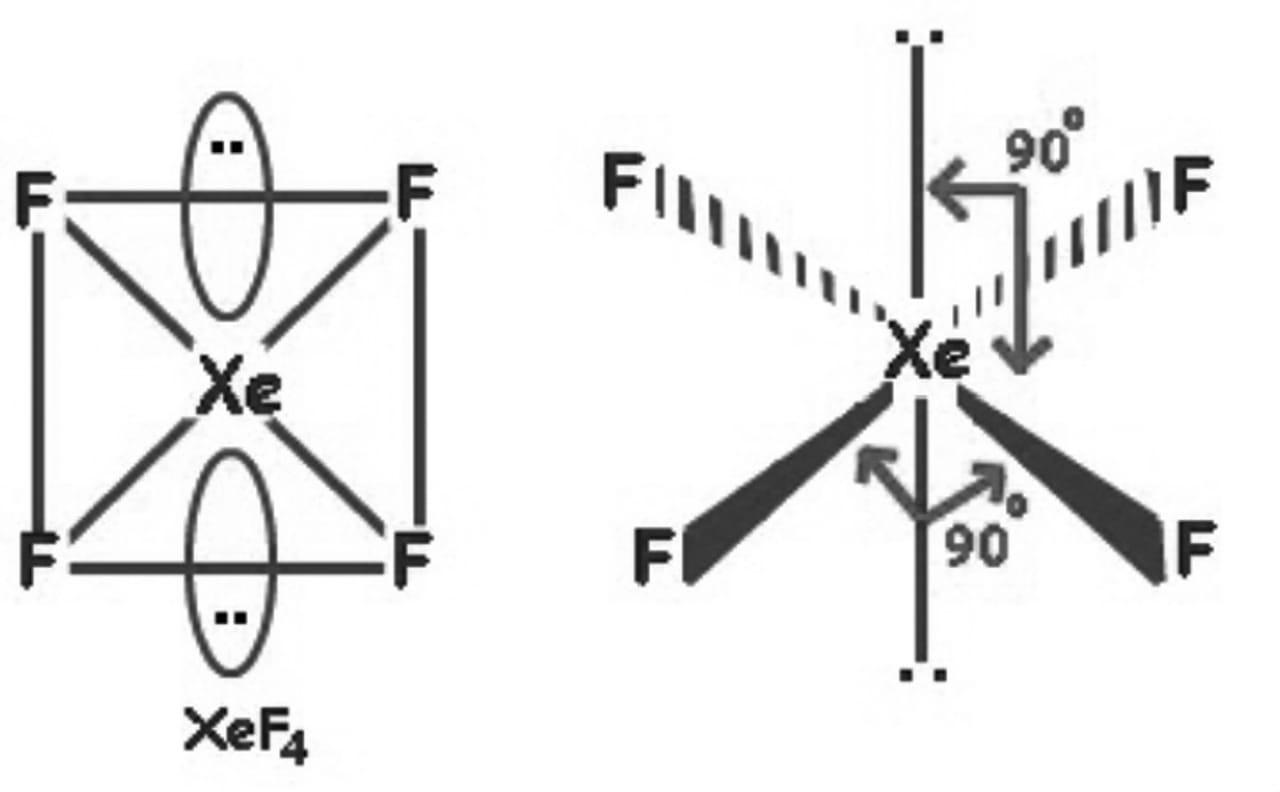
Hence, the correct answer is an option (c)- square planar.
Note: Whenever you are drawing the compound structure the number of lone pairs should also be considered. In this example also there are 4 fluorine atoms with xenon, so you could get confused between tetrahedral and square planar shape.
