Question
Question: The shape of \(\text{ }{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2 }}}\) is identical to that of: ...
The shape of H2S2 is identical to that of:
(This question has multiple correct options)
A) S2Cl2
B) O2F2
C) C2F2
D) H2O2
Solution
The bonding in the sulphur in sulphur hydrogen is different than the usual. This is particularly because of the very short distance between S−S the bond and a long-distance along with the S−H bond. Thus the two S−H bonds are arranged in the two planes perpendicular to each other. Such that the H2S2 exhibits an open book structure.
Complete answer:
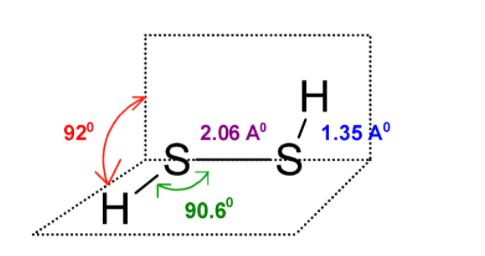
The H2S2 has a large dihedral angle that approaches 90.60 and has C2 an axis of symmetry.
The VSEPR theory is used to decide the geometry of H2S2 .
The two sulphur atoms are bonded by the disulphide linkage. Each sulphur atom is bonded to a hydrogen atom. The Lewis dot structure for the H2S2 is as follows:
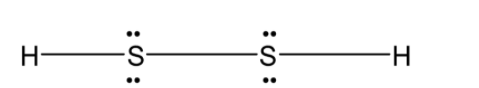
This H2S2 is an open book type structure. Since the sulphur has the two-electron pair the structure cannot be linear but it is like the hydrogen atom is in a different plane. The structure is as follows:
Let's have a look at the option.
A) S2Cl2 :
In S2Cl2 , the two sulphur atoms are forming a covalent bond and each sulphur is bonded to the two chlorine atom. The structure of the S2Cl2 is as shown below:
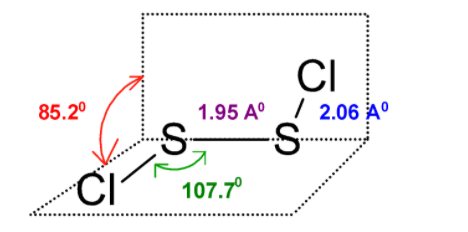
The two sulphur atoms share one bond and this is a disulphide linkage. The disulphur dichloride structure is nonlinear .Because the chlorine atom is big and reduces planarity. Thus S2Cl2 is an open book structure.
B) O2F2:
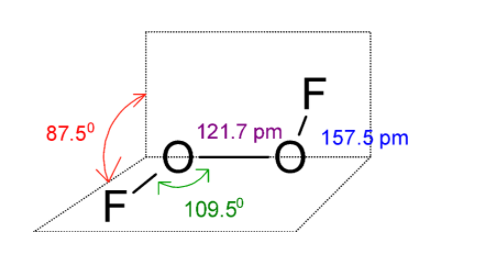
In O2F2 , oxygen atoms are bonded through peroxide linkage. This O2F2 is an open book type structure. Since the oxygen has the two-electron pair the structure cannot be linear but it is like the fluorine atom is in a different plane. The structure is as follows:
C)C2F2:
In C2F2 , the two carbon atoms are forming a covalent bond and each carbon is bonded to the two fluorine atom. The structure of the C2F2 is as shown below:
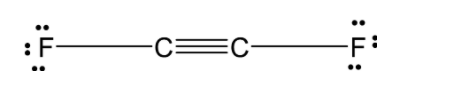
The two carbon atoms share the three bonds. Since the carbon is sp hybridized. They C2F2 have a linear structure.
D) H2O2 :
Hydrogen peroxide H2O2 is a nonplanar molecule. Two oxygen are bonded with each other forming a peroxide bond. Each oxygen atom is bonded to the hydrogen atom. Since each oxygen has two lone pairs of electrons on it. The structure is not planar. Instead of that, it is non-planar when two hydrogen atoms are in a different plane. It has an open book structure as shown below.
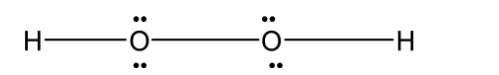
Thus the shape of H2S2 is identical to that of S2Cl2 , O2F2, H2O2 .
Hence, (A), (B), and (D) is the correct option.
Note: In general cases, the sulphur exhibits the −2 oxidation state. Sulphur in disulphide has an unusual oxidation state. In H2S2 , sulphur shows −1 an oxidation state as H is less electronegative than a sulphur atom. However in S2Cl2 sulphur is less electronegative than the chlorine atom. Thus the oxidation state of sulphur is +1 and of chlorine is −1 .
