Question
Question: The shape of \(SC{l_4}\) is best described as a: a) Square b) Tetrahedron c) Square pyramidal ...
The shape of SCl4 is best described as a:
a) Square
b) Tetrahedron
c) Square pyramidal
d) See saw
Solution
The knowledge of VSEPR theory is very important to answer these questions. Also the formula to calculate the steric number is also necessary here. We have to mention the lone pair and bond pair ofSCl4.
Complete step by step answer
VSEPR Theory was developed by Gillespie in 1957. It helps to predict and explain the molecular shapes of bond angles of molecules more appropriately.
The formula to calculate the steric number of a compound is-
SN=21[V+M−C+A] Where Vthe number of valence electrons on central atom is,M is the number of monovalent side atom, Cis the cationic charge and A is the anionic charge on the given compound. It is used to calculate the hybridisation of a compound by knowing the bond pairs and lone pairs.
Hybridisation is the process of mixing of atomic orbitals to form new orbitals, with different shapes and bond angles.
We have been given with the compound SCl4 .
In this compound the central atom is sulphur with six valence electrons as it belongs to the oxygen family and the compound has four monoatomic side atoms.
∴V=6;M=4;C=0;A=0
On substituting the values in the formula of steric number we get,
SN=21[6+4−0+0] ⇒SN=21[10] ⇒SN=5(4b.p+1l.p)
Therefore SCl4 has 4 bond pairs and 1 lone pair.
The hybridisation of SCl4 will be sp3d and the shape of the compound becomes like see saw.
The following diagram will be the correct representation:
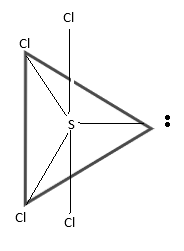
Hence,The correct option is (D).
Note: Steric numbers equal to 5 with one lone pair have see-saw structure, with two lone pairs have T-shaped geometry and with three lone pairs have linear geometry. Hence the effect of lone pairs on the shape of molecules can be easily noted.
