Question
Question: The shape of \({\rm{S}}{{\rm{O}}_{\rm{4}}}^{2 - }\) ion is: A. Square planar B. Tetrahedral C....
The shape of SO42− ion is:
A. Square planar
B. Tetrahedral
C. Trigonal bipyramidal
D. Hexagonal
Solution
If we know the number of bond pairs and lone pairs of a molecule, we can easily identify the shape of the molecule using the VSEPR theory. For that, we have to draw the Lewis structure of the molecule.
Complete step by step answer:
Let's first draw the structure of sulphate (SO42−) molecule.
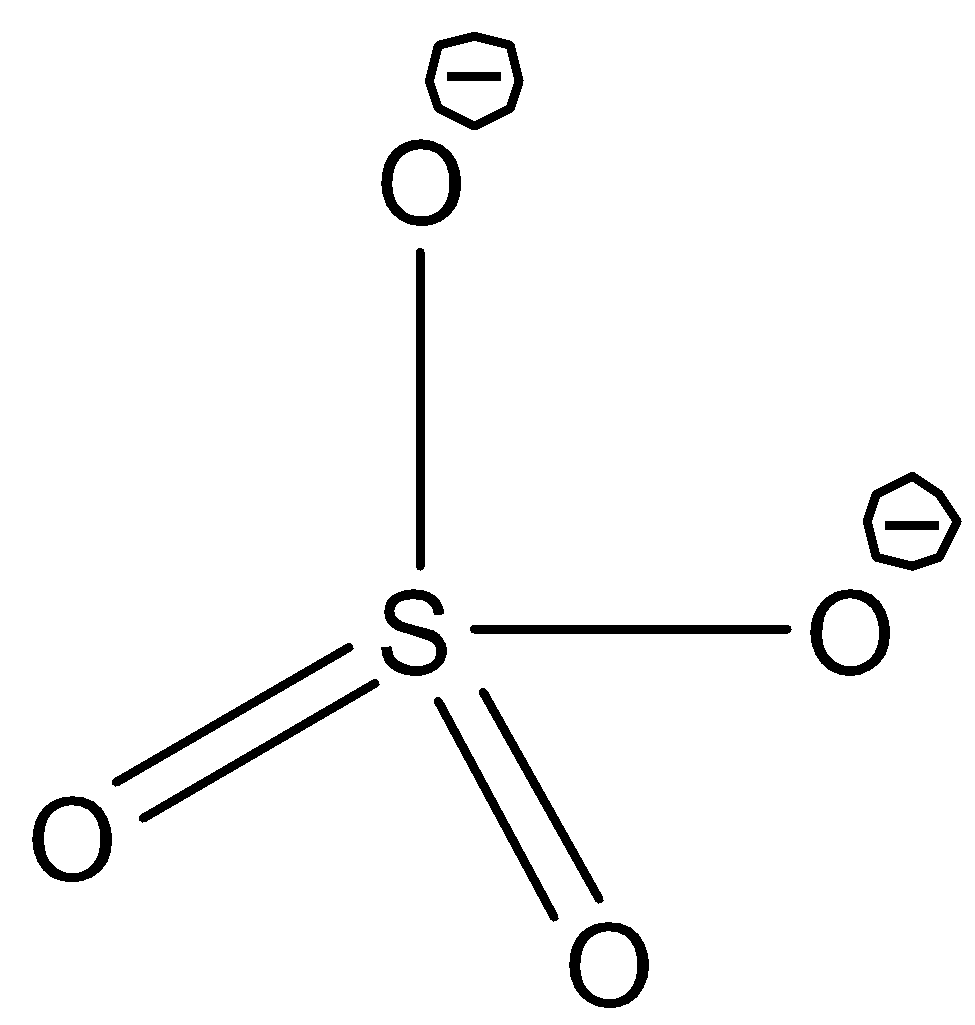
From the structure, it is observed that there are four atoms oxygen bonded to the sulphur atoms and there is no lone pair of electrons on the central atoms. There are two double bonds in the molecule. But each double bond is to be considered as a single bond. As there are four double bonds and no lone pair present. The shape of the molecule is tetrahedral.
So, the correct answer is Option B.
Additional Information:
Let's discuss VSEPR theory in detail. This theory was put forward by Gillespie and Nyholmn in the year 1957. This theory predicts the structure of the molecules in which atoms are linked together by covalent bonds only. The main points of VSEPR theory are,
- The number of electron pairs surrounding the central atom determines the shape of a molecule.
2)The electron pairs surrounding the central atom repels one another due to the negatively charged electron clouds.
3)The electron pairs in space tend to occupy such positions that they are at maximum distance apart and the repulsive interactions are minimum.
4)A multiband is considered as if it is a single bond and the electron pairs which constitute the bond may be regarded as single pairs.
Note: It is to be noted that the electron pairs existing as lone pairs cause greater repulsive interactions as compared to bonded pairs. Due to this, the order of repulsive interactions is Lone pair-lone pair>Lone pair-Bond pair>Bond pair-Bond pair.
