Question
Question: The shape of \(PC{{l}_{3}}\) molecule is: A. trigonal bipyramid B. tetrahedral C. pyramidal ...
The shape of PCl3 molecule is:
A. trigonal bipyramid
B. tetrahedral
C. pyramidal
D. square planar
Solution
PCl3 is called phosphorus trichloride, which is formed from phosphorus and chlorine. It is actually a very toxic liquid which when reacted with water releases HCl gas. The hybridisation in PCl3is sp3.
Step by step solution:
- Now let’s write the electronic configuration of phosphorus,
The atomic number of phosphorus is 15, so we can write electronic configuration as –
1s22s22p63s23p3
- Here, if we have to find the hybridisation, then it is equal to the sum of the number of sigma bonds and the number of lone pairs.
- Here, we can see that all bonds are of (P-Cl) type, we can see from the diagram drawn below that there are three P-Cl single bonds present, so the number of sigma bonds will be 3.
- And we can see here that there are 5 electrons present in valence shell that is 3s23p3, so ; two electrons are not participating in pairing , therefore the number of lone pairs will be equal to 1.
- So, we can say that hybridisation in PCl3is sp3.
- Structure of PCl3 is:
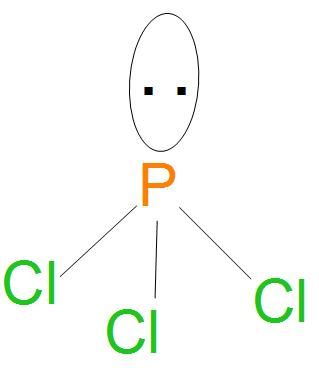
- Hence, from the structure and hybridisation of PCl3 ,we can say that the geometry is pyramidal.
Additional information:
- PCl3 is having a bond angle of approximately .
- PCl3 is found to produce , which are used in many applications, including herbicides, insecticides etc.
- It is also found to be used directly as a reagent in organic synthesis, which is also used to convert many primary as well as secondary alcohols into alkyl chlorides.
Note:
We should not get confused in between PCl3 and PCl5. PCl3 is phosphorus trichloride and is having pyramidal geometry, whereas PCl5 is phosphorus pentachloride that has trigonal bipyramidal geometry.
