Question
Question: The shape of \[{p_x}\] orbital is_____...
The shape of px orbital is_____
Solution
Electrons are sub-atomic particles that are present in the atom. These are found outside the nucleus. The region where the probability of finding an electron is maximum is called an orbital. The orbitals are of different types. p-orbital has three degenerate orbitals and the shape is a dumbbell.
Complete step by step answer:
Atoms are tiny particles consisting of sub-atomic particles called neutrons, electrons, and protons. The protons and neutrons are present inside the nucleus where the nucleus is the heavy portion in the center of the atom and the electrons are present outside the nucleus.
The region where the probability of finding an electron is maximum is known as orbitals. The region where the probability of finding an electron is maximum is known as the nodal plane or nodal region.
These orbitals are divided into different types based on the accommodation of electrons in them.
The orbitals are s, p, d, and f. The p-orbital can accommodate six electrons and is sub-divided into three types. These sub-orbitals are px,py, and pz orbitals. All these three orbitals have same energy and can be called as degenerate orbitals. The shape of these three orbitals is dumbbell shape.
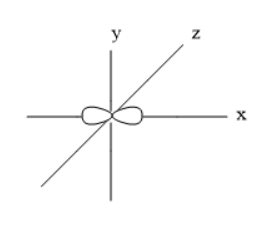
Thus, the shape of px orbital Is dumbbell.
Note:
The s-orbital can accommodate two electrons and p-orbital can accommodate six electrons and the degeneracy is three. The d-orbital can accommodate ten electrons and has the degeneracy is five and the f-orbital can accommodate fourteen electrons and has the degeneracy is seven.
