Question
Question: The shape of \( {\left( {C{H_3}} \right)_3}N \) is pyramidal because- A. Nitrogen forms three \( {...
The shape of (CH3)3N is pyramidal because-
A. Nitrogen forms three sp3 hybridized σ bonds with carbon atoms of methyl groups and there is one nonbonding electron pair.
B. Nitrogen forms three sp3 hybridized σ bonds with carbon atoms of methyl groups and fourth orbital forms π bond.
C. Nitrogen has five valencies which are arranged in pyramidal shape.
D. The unpaired electron present on nitrogen is delocalized.
Solution
Hint : The nitrogen atom has 5 valence electrons in which three valence electrons form σ bond with the three carbon atoms of the three methyl groups. The lone pair electrons are free.
Complete Step By Step Answer:
Generally sp3 hybridized molecules show tetrahedral structure but (CH3)3N is pyramidal despite having sp3 hybridization. We know that electronic configuration of nitrogen is 1s2,2s22p3 . Hence, the nitrogen atom has 5 valence electrons. Nitrogen forms three sp3 hybridized σ bonds with carbon atoms of methyl groups. Now one electron pair is left. This increases the electron density around the nitrogen atom so there is more repulsion between the electrons pairs around it. This results in a maximum bond angle which is less than that of regular tetrahedron .Due to the presence of these lone pair electrons the geometry of the molecule is distorted and it forms pyramidal shape or distorted tetrahedral. It shape is given below-
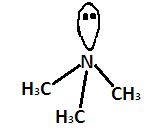
According to VSEPR theory, the shape should be tetrahedral as the electron pair will repel each other and bond angle should be 109.5∘ .
But the lone pair electrons on nitrogen repel the adjacent bonding pairs more strongly than the bonding pairs repel each other. Due to this the bond angle between the bonding pairs becomes 107.3∘
The shape changes from tetrahedral to pyramidal.
Hence, Nitrogen forms three sp3 hybridized σ bonds with carbon atoms of methyl groups and there is one nonbonding electron pair.
So option A is correct.
Note :
The structure of trimethylamine (CH3)3N is similar to the structure of ammonia in which three hybridized orbitals of nitrogen are involved in axial overlap with orbitals of carbon atoms. The lone pair on the nitrogen atom is free and the amine acts as Lewis base.
