Question
Question: The shape of gaseous \(SnC{l_2}\) is: A. Tetrahedral B. Linear C. Angular D. T-shape...
The shape of gaseous SnCl2 is:
A. Tetrahedral
B. Linear
C. Angular
D. T-shape
Solution
To determine the shape of gaseous SnCl2, the hybridization of the molecule is determined with the help of steric number. The steric number is defined as the total count of atoms joined to the central atom and the lone pair present.
Complete step by step answer:
In gaseous SnCl2, the atomic number of tin (Sn) is 50. The electronic configuration of tin is [Kr]4d105s25p2.
The total number of valence electrons is 4 out of which 2 are lone pairs and 2 are bond pairs.
The atomic number of chlorine is 17. The electronic configuration of chlorine is[Ne]3s23p5.
The valence electron in chlorine is 7.
The total number of valence electrons for SnCl2 =4+7×2=18.
The two bond pair electrons of tin form two bonds with two chlorine atoms. Thus two bonds between tin and chlorine are formed.
The steric number is 3 (two bond pairs and one lone pair).
The steric number helps to identify the hybridization of the compound. In SnCl2, three atomic orbitals of tin take part in hybridization to create three degenerate hybrid orbits. The three atomic orbitals are one s-orbital and two p-orbital. Thus, the hybridization will be sp2 and due to the presence of lone pairs the structure is bent and the resulting geometry is angular.
The structure is shown below.
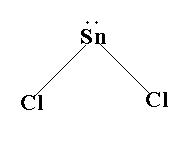
Thus, the shape of gaseous SnCl2 is angular.
So, the correct answer is Option C.
Note: When two atoms are bonded to the central atom than the geometry is linear. When three atoms are bonded to the central atom the geometry is trigonal planar. In SnCl2, if the lone pair is not observed then the geometry will be linear as two atoms are bonded to the central atom.
