Question
Question: The shape of \(Cl{O_3}^ - \) according to VSEPR model is- (A) Planar triangle (B) Pyramidal (C...
The shape of ClO3− according to VSEPR model is-
(A) Planar triangle
(B) Pyramidal
(C) Tetrahedral
(D) Square planar
Solution
Hint: This question belongs to the chapter Chemical Bonding and Molecular Structure. This question demands us to find the shape of compound ClO3−, to state the structure we need to place the formula of hybridization. By placing all the required values, we can find our answer.
Complete step by step answer:
Chlorate is a white crystalline, inorganic in nature. It is water-soluble. The material itself is noncombustible, but can form a very flammable combination with materials which are combustible, which can be explosive if the combustible material is very finely divided. Friction can ignite the mixture. Strong sulfuric acid contact may lead to fires or explosions. Spontaneous decomposition and ignition can result when mixed with ammonium salts. Long exposure to heat or fire can lead to an explosion.
The compound is ClO3−.
By placing hybridization formula, we get
⇒21 (Number of valence electron on center atom + number of monovalent atom)
⇒21(7+0+1)k { here, 7 is the valence electron on chlorine, oxygen is divalent so monovalent 0, 1 is the charge gained}
⇒21(8)=4, so we get sp3 hybridization.
4 bonds would have been made but it will be 3 bond pair and 1 lone pair
Figure –
So, the shape of the compound ClO3− would be pyramidal.
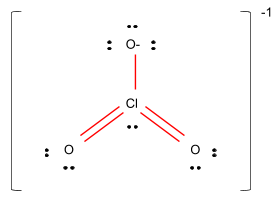
Hence, it is clear that option B is the correct option.
Note: Chlorate is a monovalent anion derived from chloric acid deprotonation. It's a mere inorganic anion and oxoanion chlorine. It is a chloric acid conjugate base.
