Question
Question: The shape of \( Cl{F_3} \) molecule is: (A) Triangular planar (B) Pyramidal (C) T-shape (D) ...
The shape of ClF3 molecule is:
(A) Triangular planar
(B) Pyramidal
(C) T-shape
(D) Trigonal bipyramidal
Solution
Hybridization: Mixing of atomic orbitals which results in the formation of new hybrid orbitals during the chemical bond formation, are known as hybridization. Hybridization occurs when electrons of s and p orbitals react to each other, then hybridization takes place to balance the energy levels.
Complete step by step solution:
First of all let us talk about hybridization and why it happens.
We know that there is a difference between the energies of s and p-orbitals. s-orbitals have less energies in comparison with p-orbitals. To balance this energy difference hybridization takes place. After the hybridization the energies of all the orbits formed are the same. For example: in sp3 hybridization one s and three p-orbitals takes place in the hybridization. Hence after hybridization four hybrid orbitals are formed which have the same energies.
Here we are given the molecule ClF3 and now we know that chlorine atom has seven electrons in its valence shell. And fluorine also has seven electrons in its valence shell. Now chlorine shares one electron with each three atoms of fluorine and has two lone pairs. So in this molecule the center atom has two lone pairs and three bond pairs. And the structure formed by this is generally trigonal bipyramidal but due to the presence of two lone pairs they repel the bond pairs so the geometry is distorted and becomes T-shape. Hence the shape of the ClF3 molecule is T-shape.
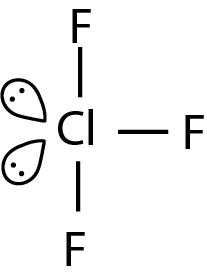
So option C is correct.
Note:
Number of hybrid orbitals formed after the hybridization is equal to the total number of orbitals taking part in the hybridization. For example if hybridization is sp2 hybridized then one s and two p- orbitals take part in the hybridization and total three hybrid orbitals will be formed after the hybridization.
