Question
Question: The shape of a molecule which has \[E{B_5}L\] molecular formula (E \( = \) central atom, B \( = \) T...
The shape of a molecule which has EB5L molecular formula (E = central atom, B = Terminal atom, L = Lone Pair)
A. Square pyramidal
B. Pentagonal planar
C. Octahedral
D. Pentagonal pyramidal
Solution
The shape of a molecule can also be determined by the geometry of a molecule. It is the arrangements of the atoms and the bonds in a molecule. The shapes of the molecules can be bent, Seesaw, trigonal pyramidal, T- Shaped, square pyramid, square planar, etc. according to the arrangement of atoms and bonds in the molecules. Also, the shapes can be determined based on the hybridization of the molecule. Various theories are there to determine the shape of the molecule (For example- VSEPR Theory, Molecular orbital theory, Linear combination of atomic orbitals).
Complete step by step answer:
The structure of EB5L molecule which has 5 bond pairs and 1 lone pair is:
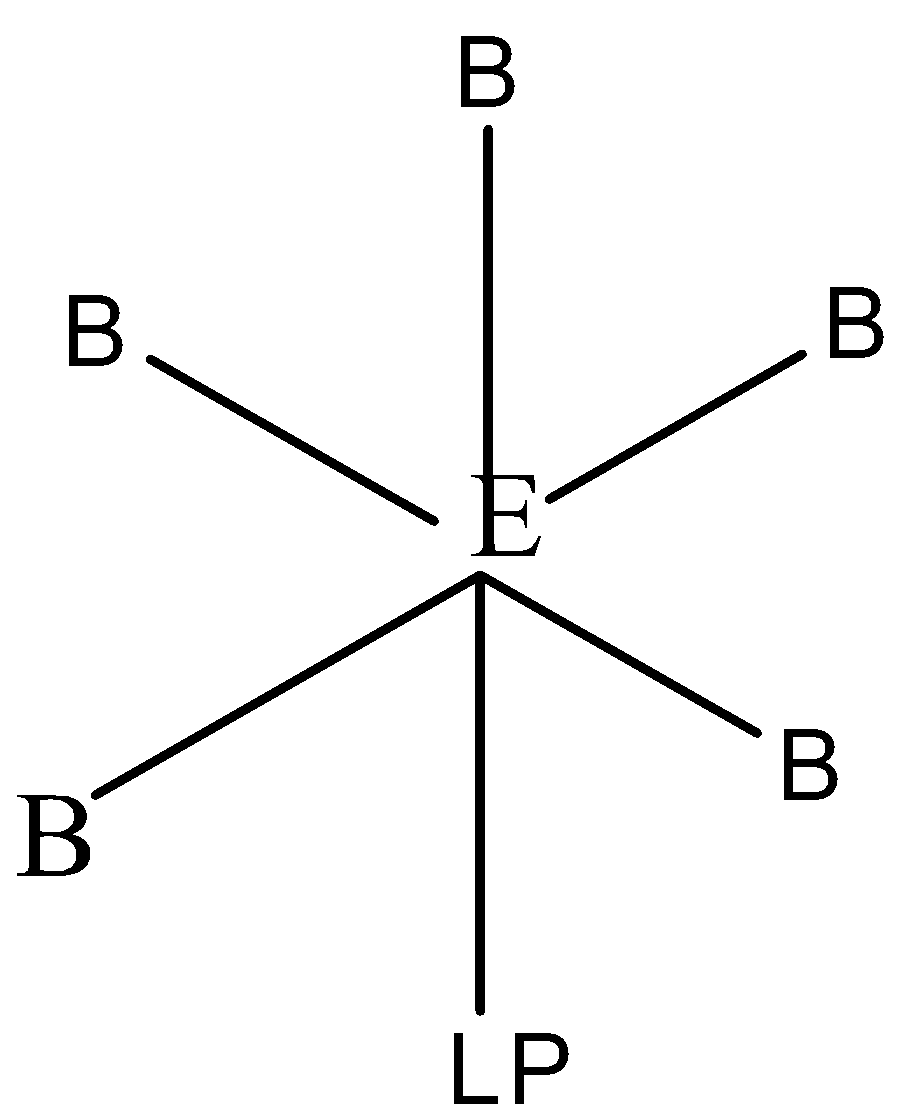
In the EB5L molecule the central atom E forms five sigma bonds with the five terminal atoms B and one lone pair (LP) which is found in one of the hybrid orbitals of the molecule.
The hybridization in EB5L molecule is sp3d2 with octahedral geometry and the shape of the molecule is square pyramidal with a bond angle of 90∘.
Example of EB5L type molecule is BrF5 (Bromine Pentafluoride).
After discussing it we can say that the shape of EB5L molecule is square pyramidal.
So, the correct answer is Option A.
Note: Hybridization is the concept of intermixing the orbitals of an atom having nearly the same energy to give exactly equivalent orbitals with the same energy, identical shapes, and symmetrical orientations in space.
There are three different types of hybridization involved in the case of carbon.
1. sp3 (tetrahedral) hybridization- When one 2s-orbital and three 2p-orbitals of excited carbon get intermixed to form four hybrid orbitals then these hybrid orbitals are known as sp3 hybrid orbitals. This phenomenon is called sp3 hybridization.
2. sp2 (Trigonal) hybridization- When 2s-orbital and two p- orbitals (2px,2py) of excited carbon atom gets intermixed to form three hybrid orbitals, then these orbitals are known as sp2 hybrid orbitals. This phenomenon is known as sp2 hybridization.
3. sp (Linear or diagonal) hybridization- When one 2s- orbital and one p-orbital (2px) of the excited carbon atom get intermixed to give two hybrid orbitals, then these hybrid orbitals are known as sp hybrid orbitals. These hybrid orbitals are known as sp hybridization.
