Question
Question: The shape of a molecule can be determined from its Lewis electron-dot diagram. Nitrogen trifluorid...
The shape of a molecule can be determined from its Lewis electron-dot diagram.
Nitrogen trifluoride (NF3) is shown in the diagram below. Which of the following choices has correctly identified both the hybridisation of the central atom and the molecular geometry ?
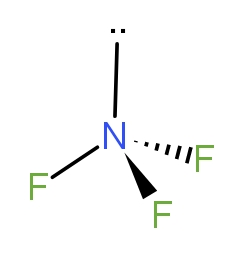
a.) sp2 trigonal planar
b.) sp3 pyramidal
c.) sp pyramidal
d.) None of these
Solution
The nitrogen trifluoride is a similar ammonia molecule. It has even hybridisation and shape of the molecule like ammonia molecule. The difference comes just in the bond length because in ammonia N-H bonds are present and in nitrogen trifluoride, N-F bonds are present.
Complete answer:
From the above figure given to us in the question, we can see that the molecule has tetrahedral geometry. As, the Nitrogen has one lone pair and lone pairs are not taken in the shape of the molecule. So, the molecule has a pyramidal shape. So, if we see the hybridisation. We can calculate that as -
Number of hybrid orbitals = Number of sigma bonds + Number of lone pairs
Number of hybrid orbitals = 3 + 1
Number of hybrid orbitals = 4
So, the hybridisation of the molecule is sp3.
Thus, the correct answer is option b.).
Note: It must be noted that Nitrogen trifluoride is colourless, non-flammable gas. It has a musty odour. It is a greenhouse gas. It has a wide number of applications. It is used in plasma etching of silicon wafers. It is used to clean PECVD chambers for the high volume production of LCDs. It is used in the hydrogen fluoride and deuterium fluoride lasers which are the type of chemical lasers.
