Question
Question: The rise of a liquid in a capillary tube is due to : A. Viscosity B. Osmosis C. Diffusion D....
The rise of a liquid in a capillary tube is due to :
A. Viscosity
B. Osmosis
C. Diffusion
D. Surface tension
Solution
Hint – The surface tension effects occur at liquid surfaces (for example in interfaces of liquid–liquid, liquid–gas, liquid–solid). Surface tension, (σ), can be defined as the force in the liquid surface normal to a line of unit length drawn to the surface. Surface tension tends to decrease with temperature and also depends on the contact fluid. Surface tension is the phenomenon involved in capillary rise and drop.
Complete step-by-step answer:
The viscosity of a liquid is a measure of its resistance to flow so this evidently cannot be the reason for the liquid rise in the capillary tube.
Also since osmosis is a process in which the molecules of a solvent pass from a solution of low concentration to a solution of high concentration via a semipermeable membrane, it cannot help liquid rise from the tube as there is no solvent. And similarly, as diffusion is defined as the movement of a substance from an area of high concentration to an area of low concentration, it too cannot be the correct answer for our question.
Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given amount. This property results from the cohesive forces between molecules at the surface of a liquid, and it causes the surface of a liquid to behave like a stretched rubber membrane. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. As a result of this high surface tension, the surface of water represents a relatively “tough skin” that can withstand considerable force without breaking.
The rise of a liquid in a capillary tube is due to surface tension which is also known as capillary action.
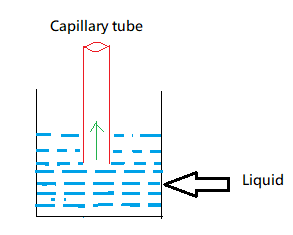
Since the liquid is in contact with the capillary tube as we can see in the above figure, it starts experiencing surface tension. That means the capillary tube starts experiencing an upward force. Due to this upward force the liquid starts rising upwards. And the liquid keeps on rising till the surface tension is balanced by the weight of liquid
Hence, the correct option for the above question is – option (D) ie. Surface tension.
Note- One of the applications of the capillary action is used to draw blood. Many medical tests require extracting a small amount of blood from the person’s body. This procedure can be easily done using the knowledge of capillary action, the ability of a liquid to flow up a small tube against gravity. Also another application of capillary action is the soaps and detergents reduce surface tension of water. Hence, they are often used for cleaning clothes and utensils.
