Question
Question: The reversible expansion of an ideal gas under adiabatic and isothermal conditions is shown in the f...
The reversible expansion of an ideal gas under adiabatic and isothermal conditions is shown in the figure. Which of the following statement(s) is(are) correct?
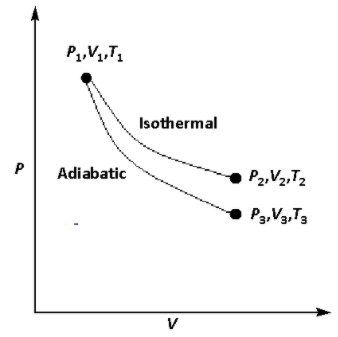
A.T1=T2
B.T3>T1
C.Wisothermal>Wadiabatic
D.ΔUisothermal>ΔUadiabatic
Solution
We know that the process in which the temperature of the system remains the same is known as an isothermal process. In an isothermal process, ΔT=0. Also, we know that the process in which heat of the system remains constant is known as adiabatic process. In an adiabatic process, Δq=0.
Complete step by step solution: As we know in an isothermal process, the temperature of the system remains constant throughout the process.
In the given figure, in the isothermal curve, the initial temperature is T1 and the final temperature is T2. As the process is isothermal, the temperature will not change. Thus,
T1=T2
Thus, option (A) is correct.
As we know in an adiabatic process, the heat of the system remains constant throughout the process.
In the given figure, in the adiabatic curve, the initial temperature is T1 and the final temperature is T3. As the process is adiabatic, the final temperature is lower than the initial temperature. Thus,
T1>T3
Thus, option (B) is not correct.
In an isothermal process, the change in internal energy is zero. Thus,
ΔUisothermal=0
This internal energy gets converted to work Wisothermal.
In an adiabatic process, the change in heat is always zero. Thus,
Δq=0
Thus, the work done in an adiabatic process is always negative.
Thus, we can say that the work done in the isothermal process is higher than the work done in an adiabatic process. Thus,
Wisothermal>Wadiabatic
Thus, option (C) is correct.
In an isothermal process, the change in internal energy is zero. But in adiabatic processes, the change in internal energy is always negative. Thus,
ΔUisothermal>ΔUadiabatic
Thus, option (D) is correct.
Thus, the correct options are (A), (C) and (D).
Note: We are given a reversible expansion of gas. The process which can be reversed by change is known as a reversible process. In a reversible process, equilibrium exists between states. The process that cannot be reversed is known as irreversible process.
