Question
Question: The relative reactivity of \({{1}^{\circ }}H\), \({{2}^{\circ }}H\), and \({{3}^{\circ }}H\) in brom...
The relative reactivity of 1∘H, 2∘H, and 3∘H in bromination reaction has been found to be 1 : 82 : 1600 respectively. In the reaction the percentage yield of the products (A) and (B) are expected to be:
(a)- 99.4%, 0.6%
(b)- 50%, 50%
(c)- 0.6%, 99.4%
(d)- 80%, 20%
Solution
The relative reactivity given is 1: 82: 1600 of primary, secondary, and tertiary hydrogen means the amount of the tertiary compound will be very high as compared to the amount of the primary compound.
Complete Solution :
Bromination of alkane means, one hydrogen atom from the alkane is substituted with one bromine atom. This process leads by the formation of a carbocation in the alkane and then the negative bromine attacks the positive carbon atom. We know that there are three types of hydrogen atoms in the alkane, i.e., primary, secondary, and tertiary. The relative reactivity given is 1: 82: 1600 of primary, secondary, and tertiary hydrogen means the amount of the tertiary compound will be very high as compared to the amount of the primary compound.
So, in the given reactant:
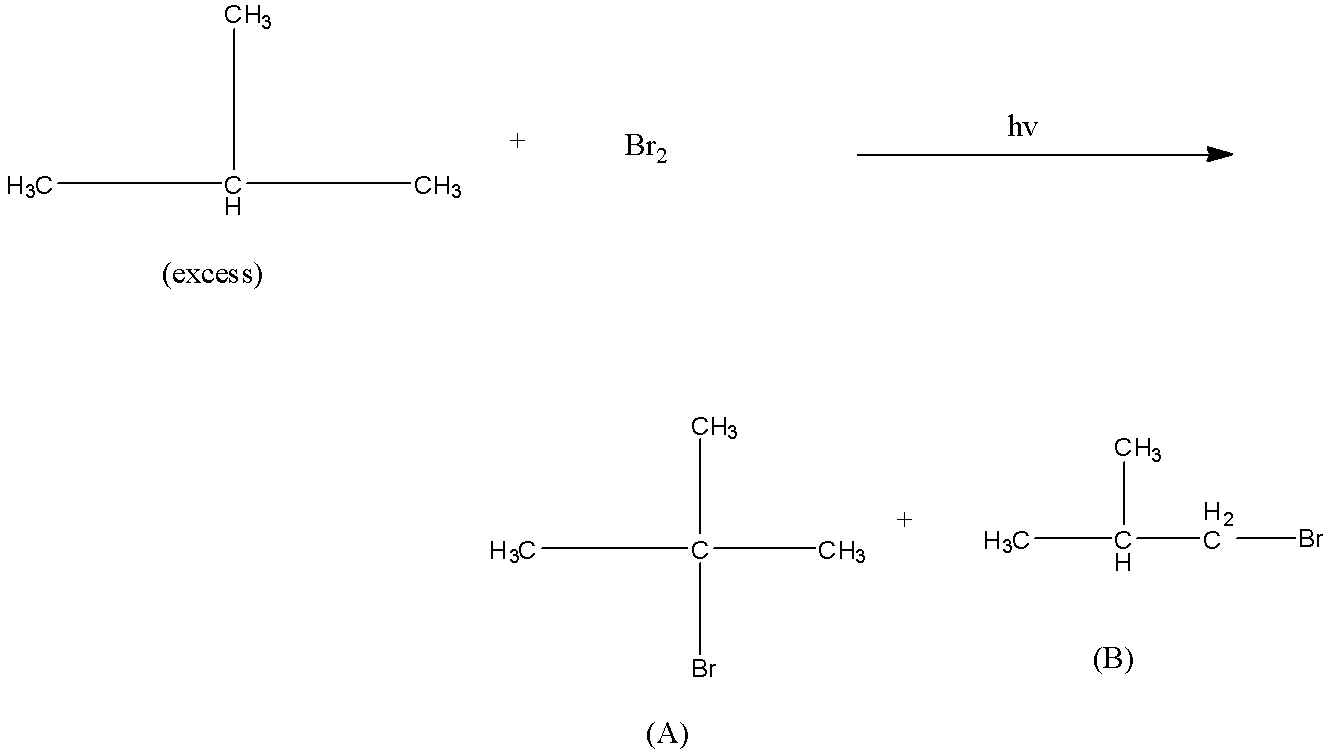
There are two types of hydrogen atoms, i.e., tertiary and primary hydrogen. Therefore, there will be the formation of two products, i.e., first, by the substitution of the tertiary hydrogen and second, by the substitution of the primary hydrogen. The product formed by the substitution of the tertiary hydrogen will be major and according to the reactivity, its formation will be 99.4% and the rest will be 0.6%. Hence, in the question product (A) will be 99.4% and product (B) will be 0.6%.
So, the correct answer is “Option A”.
Note: Same as the bromination, when chlorination is done on the alkane then the relative reactivity of primary, secondary, and tertiary product will be 1: 3.8: 5. So, bromination is easier than chlorination.
