Question
Question: The reagent used in the Wolff-Kushner reduction is : (A). \[{{H}_{2}}N-N{{H}_{2}}/KOH\] (B). \[{...
The reagent used in the Wolff-Kushner reduction is :
(A). H2N−NH2/KOH
(B). H2/Ni
(C). Sn/HCI
(D). LiAIH4
Solution
Wolff Kushner reduction is a type of reduction in which a carbonyl group is converted into a methyl group . This reduction was discovered by N- Kushner in 1911 and Ludwig Wolff in 1912 . That’s why this reduction process is known as Wolff Kushner reduction.
Complete step by step answer:
Wolff Kushner reduction is a reduction process used in organic chemistry to convert the carbonyl functionalities into methyl groups. In this reaction, the carbonyl group is treated with hydrogen to give the initial product as nitrogen. The hydrogen is reacted with KOH/glycal to remove N2 and to give the final methylene group as the product.
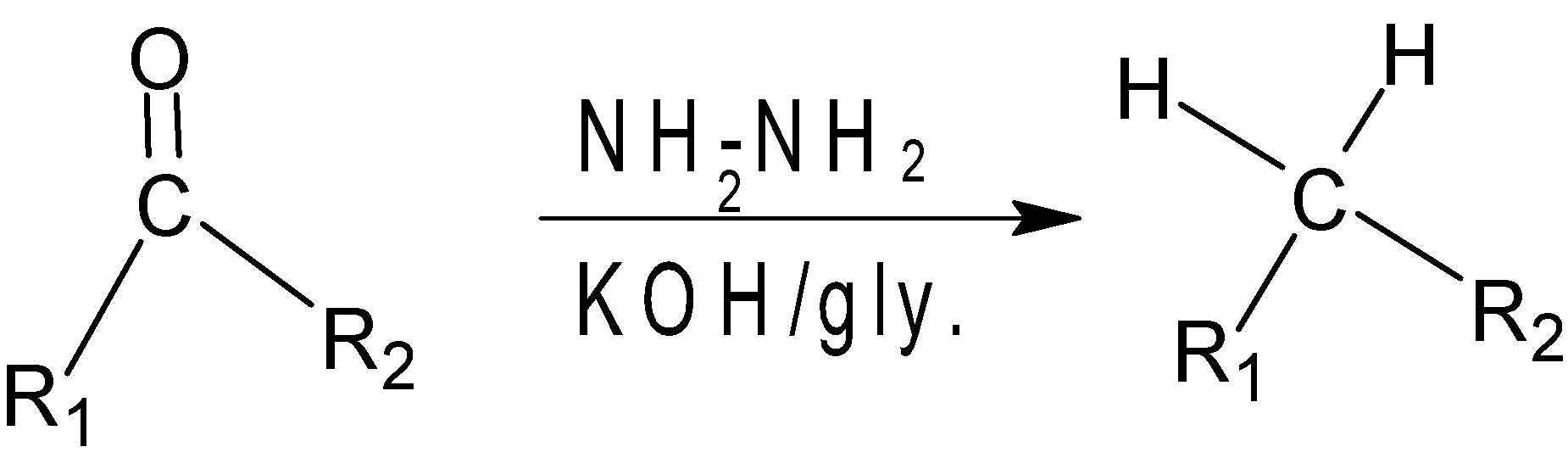
Mechanism of the reaction:-
Step A :-
The carbonyl compound such as aldehyde or ketone is treated with hydrazine which results in information of hydrazine.
That is,
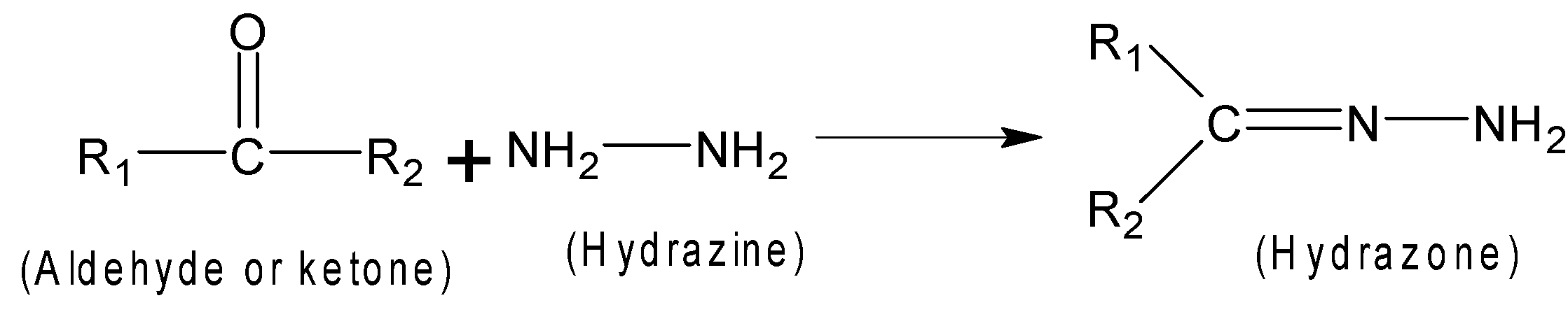
Here R1 and R2 can be any alkyl group .
Step B :-
Now the hydrazone is treated with KOH , which releases OH−ions which deprotonates the hydrazone. Due to this a double bond is formed between two adjacent nitrogen atoms. The released proton combines with OH− to form water.

(hydrazone )
Step C : The oxygen is more electronegative and withdraw electrons towards it than carbon, so the carbon gets protonated.
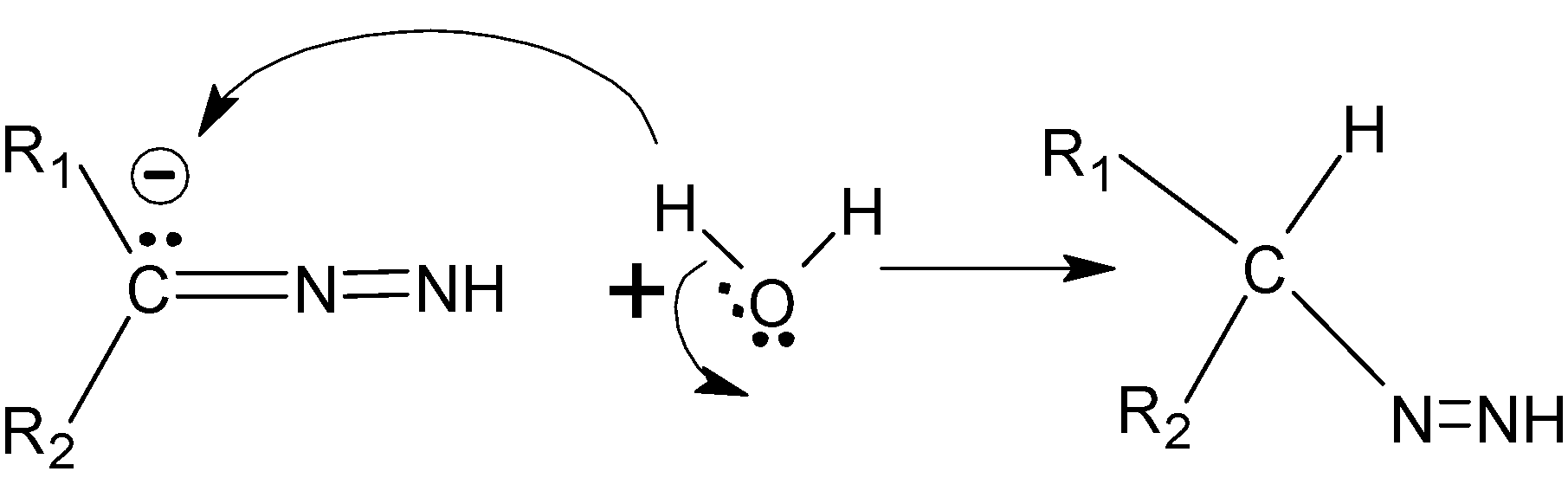
Step D :- The nitrogen groups are deprotonated again by the effect of KOH, and this time it forms triple bond with nitrogen atom which leads to the removal of N2 from the compound.
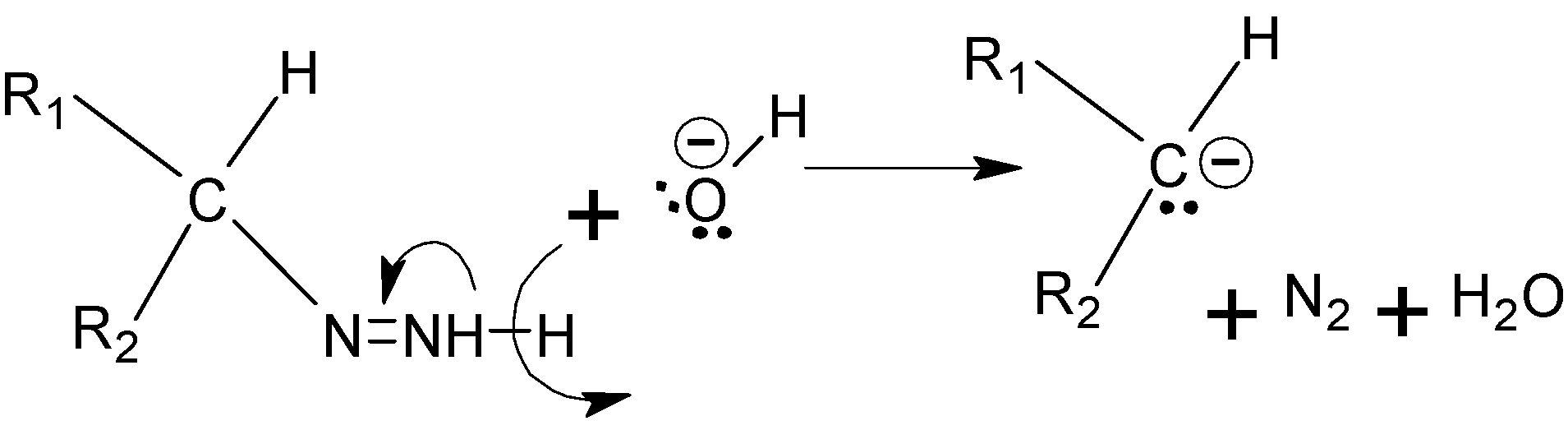
Step E :- Now the carbon again gets protonated from the water to give the final alkane.
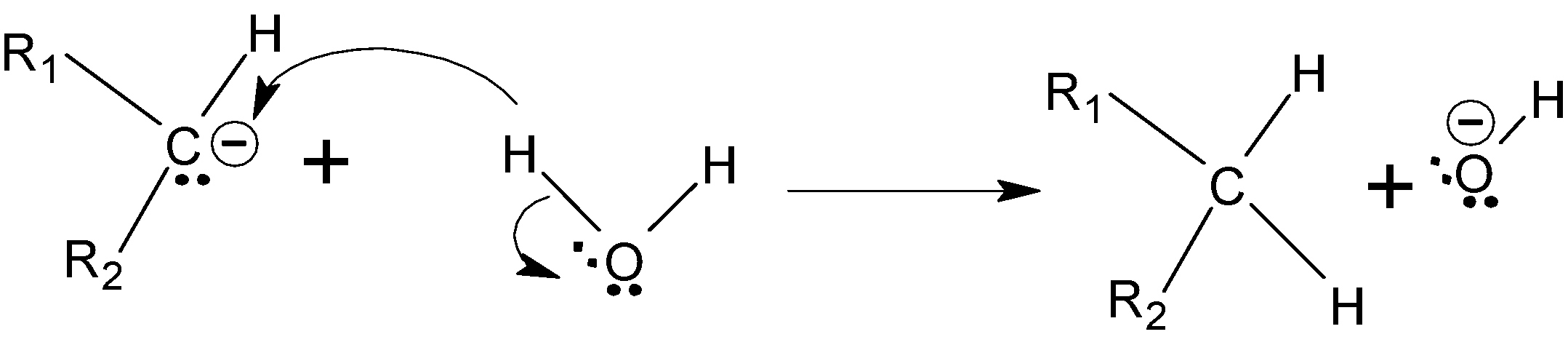
Hence we got to know that H2N−NH2 and KOH is used in wolff Kushner reduction.
So, the correct answer is Option A.
Note: We can use any base other than KOH in this Wolff Kushner reduction because we only needs OH− ion in its mechanism which can be given by any base. The Wolff Kushner reduction has been modified into various techniques such as the Huang Minlon modification that offer reduced reaction time and the achievement of higher temperatures but requires distillation.
