Question
Question: The reagent commonly used to determine hardness of water titrimetrically is: A.Oxalic acid B.So...
The reagent commonly used to determine hardness of water titrimetrically is:
A.Oxalic acid
B.Sodium citrate
C.Disodium salt of EDTA
D.Sodium carbonate
Solution
In volumetric analysis or complexometric titrations, a number of metal ions react completely with polydentate ligands at an appropriate pH to form complexes.
The hardness of water which is due to the presence of calcium and magnesium ions can thus be estimated by these complexometric titrations.
Complete step by step answer:
The solutions of metal ions in case of complexometric titrations or volumetric analysis can be titrated against the solutions of the polydentate ligands in the presence of a suitable buffer and the end point can be detected by the use of a suitable indicator.
Polydentate ligands are those ligands which contain more than two donor atoms or coordinating groups in their molecules which can coordinate to the central metal atom or ion.
The most common polydentate ligand is the hexadentate ligand called edta or the ethylenediaminetetraacetic acid ligand. It is also abbreviated as EDTA. It has a total of 6 donor atoms. There are two nitrogen atoms and four oxygen atoms of the four carboxylic acid groups which are all capable of bonding to the metal atom. It is usually represented as H4Y .
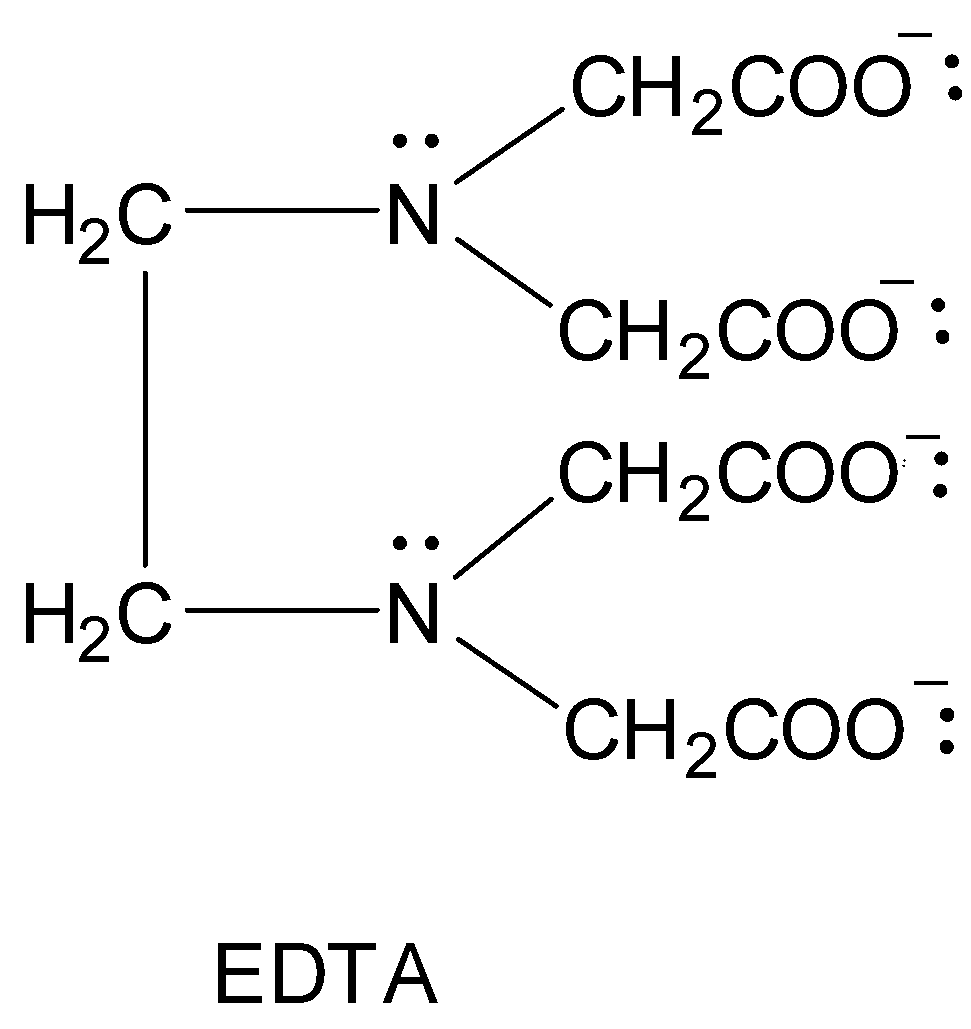
Its disodium salt is most commonly used in volumetric titrations as it has better solubility in water than the acid. It is represented as Na2H2Y . It ionizes as follows.
Na2H2Y→2Na++H2Y2−
Now, hard water contains calcium and magnesium metal ions (in general, M2 + ) and they can undergo reaction with EDTA as follows.
M2 + +H2Y2−→MY2−+2H+
Thus, this helps in reducing the concentration of the calcium and magnesium ions in water and is responsible for the purification of water.
So, the correct option is C.
Note:
-EDTA is used intravenously to treat lead poisoning and brain damage, to remove copper in patients suffering from Wilson’s disease.
-It is also used for reducing the level of calcium in people who have a high level of calcium.
-It is also used in eyedrops to treat the calcium deposits in the eye.
