Question
Question: The reaction sequence given below is carried out with 16 moles of X. The yield of the major product ...
The reaction sequence given below is carried out with 16 moles of X. The yield of the major product in each step is given below the product in parentheses. The amount (in grams) of S produced is ______.
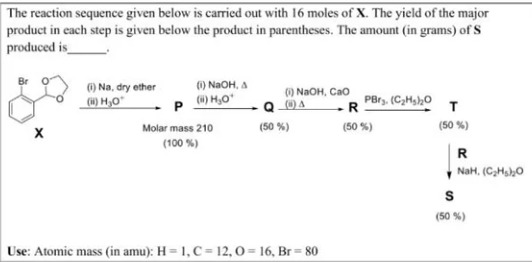
1056
Solution
The overall reaction is:
C2H5OH⟶CH3CHO⟶CH3COOH⟶CH3COOC2H5⟶CH3CONH2
Moles of CH3CHO formed =16×0.6=9.6
Moles of CH3COOH formed =9.6×0.8=7.68
Moles of CH3COOC2H5 formed =7.68×0.5=3.84
Moles of CH3CONH2 formed =3.84×0.9=3.456
Mass of CH3CONH2=3.456×59=203.904g
The second reaction is:
C2H5OH⟶C2H5Br⟶C2H5OC2H5
Moles of C2H5Br formed =16×0.7=11.2
Moles of C2H5OC2H5 formed =11.2×0.75=8.4
Mass of C2H5OC2H5=8.4×74=621.6g
The third reaction is:
C2H5OH⟶CH2=CH2⟶CH2Br−CH2Br
Moles of CH2=CH2 formed =16×0.85=13.6
Moles of CH2Br−CH2Br formed =13.6×0.4=5.44
Mass of CH2Br−CH2Br=5.44×188=1022.72g
Total mass of products =203.904+621.6+1022.72=1848.224g
The fourth reaction is:
C2H5OH⟶CH3CHO⟶CH3COOH⟶(CH3CO)2O⟶CH3COCl⟶CH3CO−N(CH3)2
Moles of CH3CHO formed =16×0.6=9.6
Moles of CH3COOH formed =9.6×0.8=7.68
Moles of (CH3CO)2O formed =7.68×0.5=3.84
Moles of CH3COCl formed =3.84×0.9=3.456
Moles of CH3CO−N(CH3)2 formed =3.456×0.6=2.0736
Mass of CH3CO−N(CH3)2=2.0736×87=180.39g
Overall reaction:
C2H5OH⟶CH2=CH2⟶C2H4Br2⟶CH≡CH⟶AgC≡CAg⟶C+Ag
16⟶16×0.85⟶16×0.85×0.4⟶16×0.85×0.4×0.6⟶16×0.85×0.4×0.6×0.7⟶16×0.85×0.4×0.6×0.7×0.8
Moles of Ag=16×0.85×0.4×0.6×0.7×0.8=1.8304
Mass of Ag=1.8304×108=197.6832g
Moles of C=1.8304/2=0.9152
Mass of C=0.9152×12=10.9824g
Moles of S=16×0.75×0.8×1.1=10.56
Mass of S=10.56×100=1056
