Question
Question: The reaction of t-butyl bromide with sodium methoxide mainly produces: A. isobutane B. isobutyle...
The reaction of t-butyl bromide with sodium methoxide mainly produces:
A. isobutane
B. isobutylene
C. t-butyl methyl ether
D. sodium tert-butoxide
Solution
Think about the standard name reaction mechanism that involves the reaction of an alkyl halide and alkoxide ion. Consider mechanisms for primary, secondary, as well as tertiary alkyl halides.
Complete step by step solution:
This kind of reaction is known as the Williamson synthesis. The alkyl halide reacts with a metal alkoxide. It forms an ether is the alkyl halide is primary and an alkene if the alkyl halide is secondary or tertiary.
For a primary alkyl halide, by the SN2 mechanism, the alkoxide ion attacks the carbon attached to the halogen atom. This halogen atom is then displaced and an ether is formed. This reaction mechanism does not favour the formation of bulky ethers. Hence, if the alkyl halide is secondary or tertiary, the reaction that happens is an elimination reaction instead of a displacement reaction due to steric hindrance.
The elimination occurs by the E1cB mechanism of elimination and dehydrohalogenation takes place. Thus, an alkene is formed. The reaction that occurs will be:
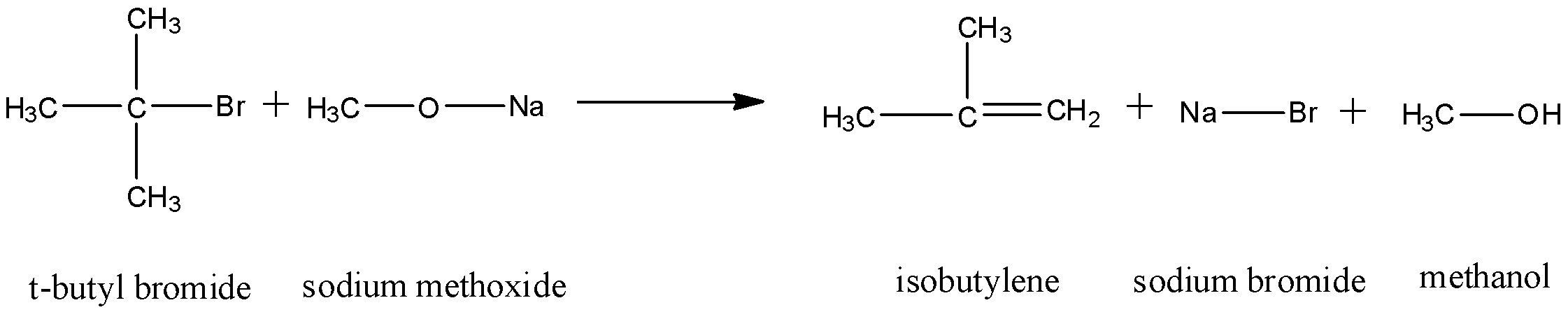
Thus, as we can see, dehydrohalogenation occurs on the tertiary carbon and an adjacent carbon. A double bond forms between them and formation of an alkene occurs.
Hence, the answer is ‘B. isobutylene’
Note: Please do not get confused due to the fact that this is a Williamson synthesis. Remember that only primary alkyl halides form ethers. Because of this, do not blindly mark your answer as ‘C. t-butyl methyl ether’. Always check if the alkyl halide is primary, secondary, or tertiary before working out the reaction and marking the answer.
