Question
Question: The reaction of propene with \(HOCl\) proceeds via the addition of: (A) \(C{{l}^{+}}\) and \(O{{H...
The reaction of propene with HOCl proceeds via the addition of:
(A) Cl+ and OH− in a single step
(B) Cl+in the first step
(C) H+ in the first step
(D) OH−in the first step
Solution
Propene is a member of group alkene in which a double bond is present. The alkenes undergo Electrophilic Addition reaction, in which the first step is the attack of an electrophile or atom/molecule with a positive charge.
Complete step by step solution:
Propene is a compound of group alkene in which a double bond is present at terminal carbon and its formula isCH3−CH=CH2. The alkenes show an Electrophilic Addition reaction, which means that the double bond gets converted into a single bond by the addition of electrophiles to the carbon atoms of the double bond.
So, HOClis called hypochlorous acid in which the hydroxyl is the negative part and the chloride ion is the positive part, this is due to the higher electronegativity of the oxygen than chlorine.
The reaction takes place as the chlorine reacts with water to form hypochlorous acid. Now, this hypochlorous acid splits into two ions and these two ions get attached with the double bond in the propene forming 1-Chloropropan-2-ol. The reactions are given below:
Cl2+H2O→HOCl+HCl
CH3−CH=CH2+HOCl→CH3−CH(OH)−CH2(Cl)+HCl
The reaction occurs in two steps. In the first step, the chlorine adds slowly to the double bond, and in the second step rapid nucleophile attack byH2O. The mechanism is given below:
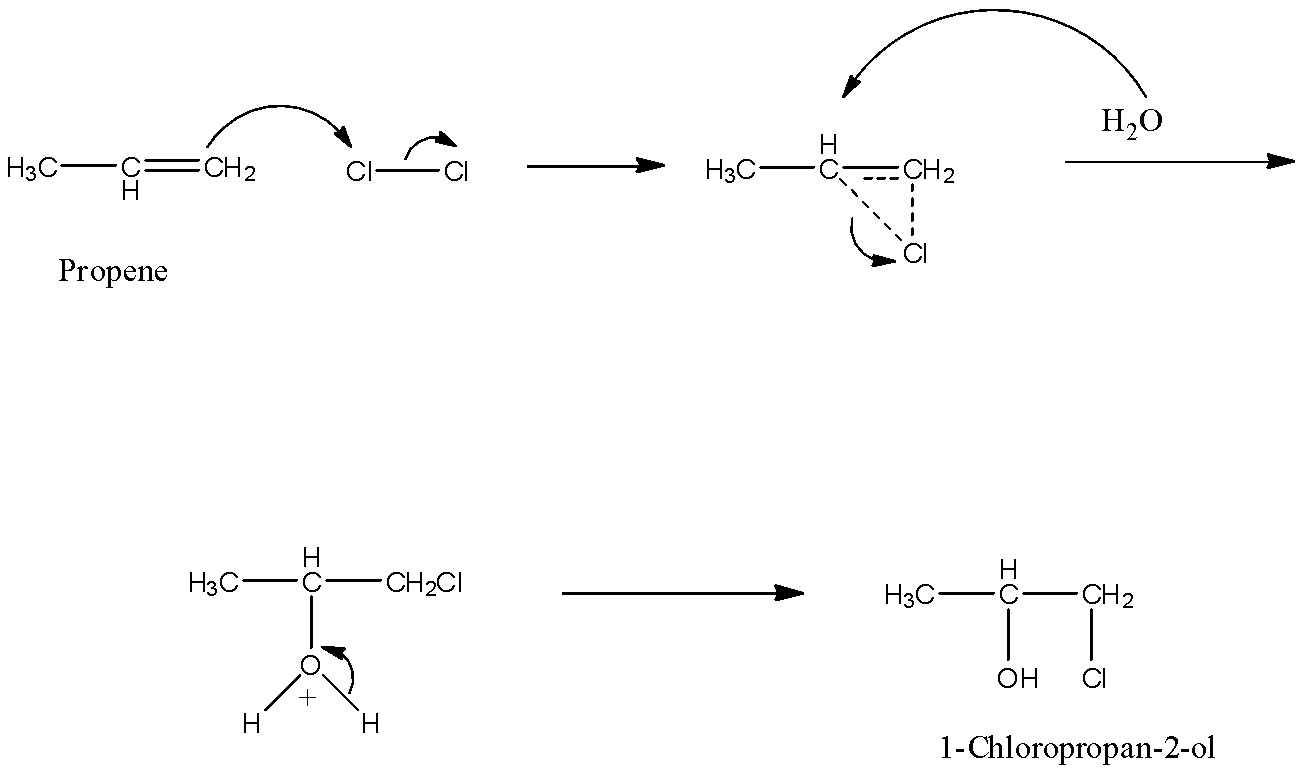
So, the correct answer is an option (B)- Cl+ in the first step.
Note: Fluorine doesn't give this reaction. And the reactivity of the halogen towards this reaction decreases as we move down the group, i.e., chlorine > bromine > iodine. Mostly chlorine and bromine give this reaction.
