Question
Question: The reaction of methyl trichloroacetate \((C{l_3}CC{O_2}Me)\) with, sodium methoxide \(\left( {NaOMe...
The reaction of methyl trichloroacetate (Cl3CCO2Me) with, sodium methoxide (NaOMe) generates:
A.Carbocation
B.Carbene
C.Carbanion
D.Carbon radical
Solution
In any chemical reaction 2 reactants react with each other to form product and formation of product depends upon the type of reactants that are reacting and presence of atoms or molecules in the reactants.
Step by step answer: When methyl trichloroacetate reacts with sodium methoxide then the formed product is carbine. And the mechanism of the reaction which takes place between these both reactants is shown as follow:
Firstly negatively charged oxygen of sodium methoxide (NaOMe)attack on the carbonyl carbon of methyl trichloroacetate (Cl3CCO2Me)because it bears partial positive charge.
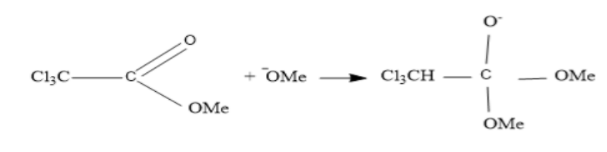
Now the formed product on the above step do rearrangement to form trichloromethane anion and by product.
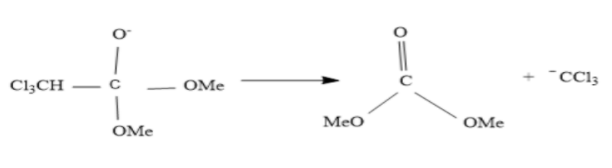
Now trichloromethane anion changes to carbene and chloride ions.
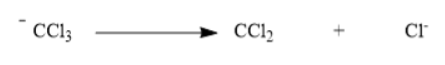
Hence the correct option is B.
Additional information: Carbene is a neutral molecule in which carbon atoms form two bonds and two unshared electrons are also present, and these two electrons are not lone pairs of electrons. Cabinet is generally also known as methylene.
In the option carbocation name is also present, which is a species of carbon in which carbon carries position charge and it comes under the category of cation.
In the option carbanion name is also present, which is a species of carbon in which carbon carries negative charge and it comes under the category of anion.
Note: in the given question you may go wrong during the formation of bonds, so for neglecting this type of mistake always keep in mind that negatively charged part of one reactant attack on the positive part of the other reactant.
