Question
Question: The reaction of cyanamide, NH₂CN(s) with oxygen was run in a bomb calorimeter and ΔU was found to be...
The reaction of cyanamide, NH₂CN(s) with oxygen was run in a bomb calorimeter and ΔU was found to be –742.24 kJ mol⁻¹. The magnitude of ΔH₂₉₈ for the reaction
NH₂CN(s) + 23O₂(g) → N₂(g) + O₂(g) + H₂O(l) is ____ kJ.
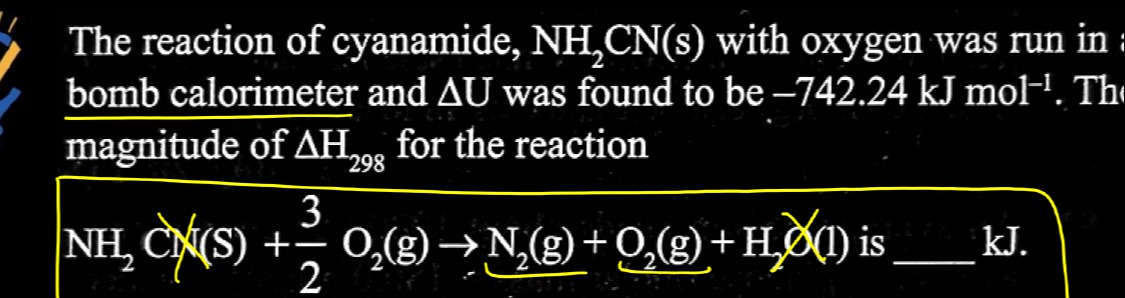
A
741.00
B
742.24
C
743.48
D
740.00
Answer
741.00
Explanation
Solution
The relationship between enthalpy change (ΔH) and internal energy change (ΔU) is given by ΔH=ΔU+ΔngRT. For the given reaction, Δng=(moles of gaseous products)−(moles of gaseous reactants)=(1+1)−23=2−1.5=0.5 mol. Given ΔU=−742.24 kJ mol⁻¹, T=298 K, and R=8.314×10−3 kJ mol⁻¹ K⁻¹. ΔH=−742.24 kJ mol−1+(0.5 mol)(8.314×10−3 kJ mol−1 K−1)(298 K) ΔH=−742.24 kJ mol−1+1.238786 kJ mol−1 ΔH≈−741.001214 kJ mol−1. The magnitude of ΔH is approximately 741.00 kJ.
