Question
Question: The reaction of aniline with chloroform under alkaline conditions lead to the formation of: A) Phe...
The reaction of aniline with chloroform under alkaline conditions lead to the formation of:
A) Phenyl cyanide
B) Phenyl isonitrile
C) Phenyl cyanate
D) Phenyl isocyanate
Solution
Aliphatic and the aromatic primary amines when warmed with chloroform with the chloroform and an alcoholic solution of KOH , form isocyanides or carbylamines which have a very unpleasant or foul smell. The general reaction of the amine is given as follows,
R−NH2 1oamine +CHCl3+3KOH(alc.)warmR−NCAlkyl isocyanide+RNC+3H2O
Complete step by step answer:
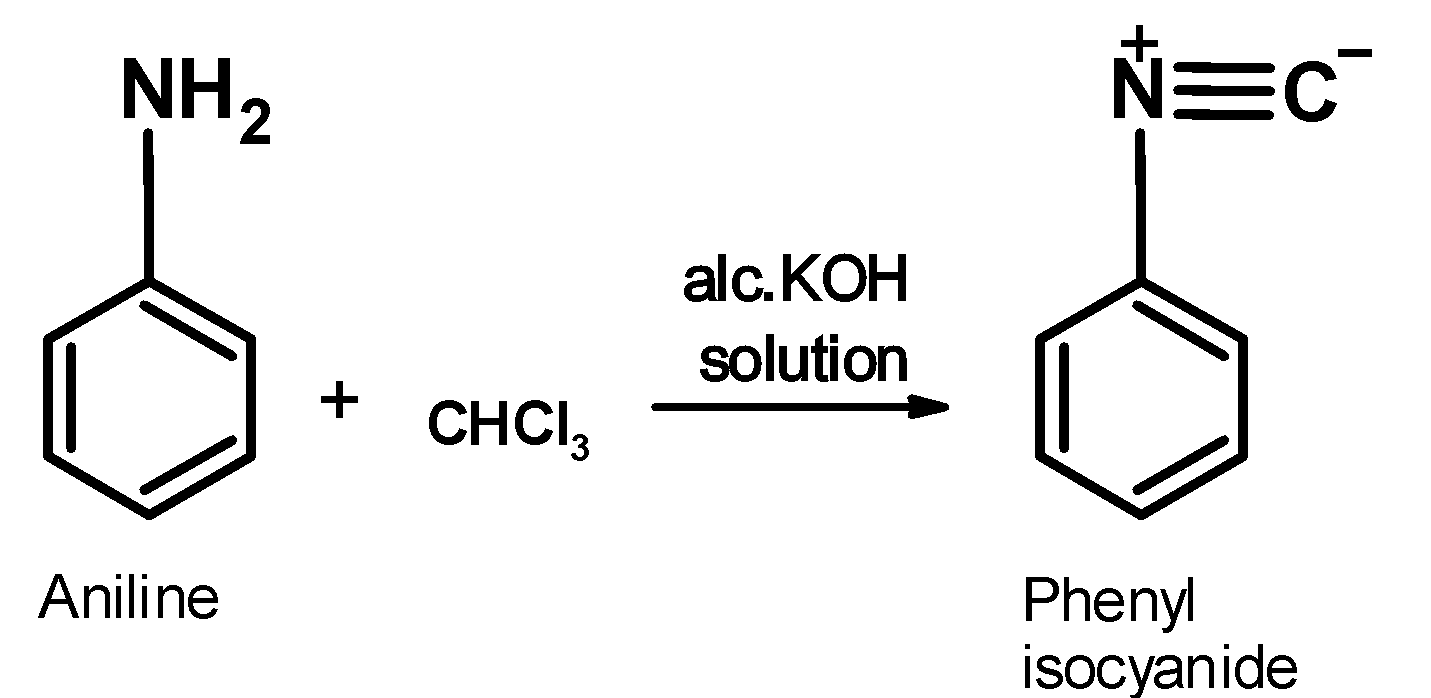
Aniline is an aromatic amine. its general structural formula is Ar−NH2 . It contains the amine −NH2 group. When aniline is warmed with the chloroform in the alcoholic alkaline solution, it generates an organic product which has a distinct bad or foul sell .this compound is an isonitrile .the reaction of aniline is as shown below,
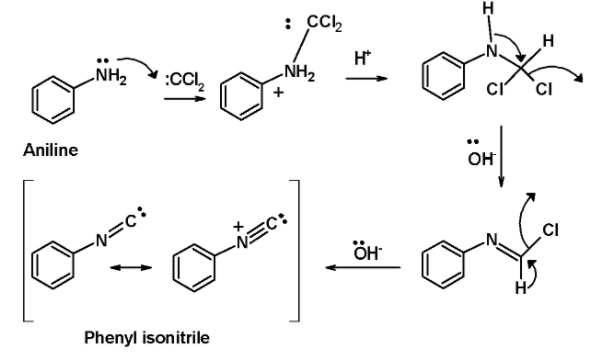
The mechanist pathways for the carbylamine test is as shown below,
In step 1, the dichlorocarbene derived from the chloroform is attacked by the lone pair of electrons on the nitrogen atom of the amine. This generates an intermediate by the dehydrohalogenation of the chloroform.
In the next step, the base i.e. potassium hydroxide in the alcoholic solution abstracts two protons from the intermediates in the successive step. This generates an isocyanides product. The entire mechanistic pathway for the reaction is as shown below,
Aromatic amine ‘aniline’ reacts with the chloroform in an alkaline solution leading to the formation of phenyl isocyanides or phenyl isonitrile.
Hence, (B) is the correct option.
Note: Note that, the secondary or the tertiary amines do not give the carbylamine test. This test is used to distinguish between the primary amines from the secondary and the tertiary amines. Isocyanides are also known as the isonitrile same as we call cyanide and nitrile. Remember that here the position of attachment has a significant role in determining the name. It attaches through N thus known as isonitrile.
