Question
Question: The reaction intermediate produced by hemolytic cleavage of a bond is called: A.Carbene B.Carboc...
The reaction intermediate produced by hemolytic cleavage of a bond is called:
A.Carbene
B.Carbocation
C.Carbanion
D.Free radical
Solution
Homolytic cleavage is the breaking of a covalent bond in such a way that each fragment gets one of the shared electrons and each atom shares electrons with itself.
Complete step by step answer:
The reaction intermediate produced by homolytic cleavage of bonds is called free radical.
The covalent bond is cleaved in such a manner that each atom shares electrons with itself and free radical is formed.
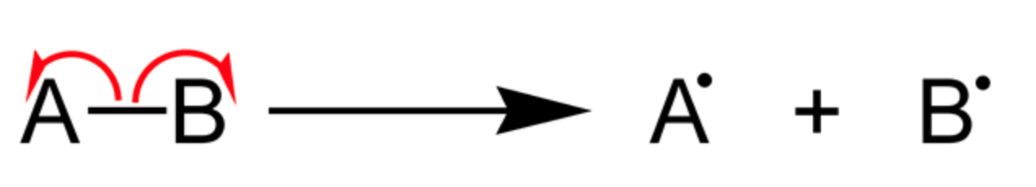
Free radicals are the reaction intermediates formed containing carbon such that carbon gets an unpaired electron. In this process each atom takes away one of the two electrons forming a single covalent bond. It will further produce two new species.
This process is also known as radical fission and the energy required for homolytic cleavage is bond-dissociation energy.
Hence, option D is correct.
Note:
Free radicals, molecules (or atoms) having one or more unpaired electrons are recognized as important short-lived, highly reactive intermediates in a variety of organic, and some inorganic reactions. These are derived either from normal metabolic processes in the human body or from external sources such as exposure to X-rays, ozone, industrial chemicals.
