Question
Question: The reaction $2NO_2CI \rightarrow 2NO_2 + Cl_2$ The mechanism is, $1st step: NO_2Cl \xrightarrow{k_...
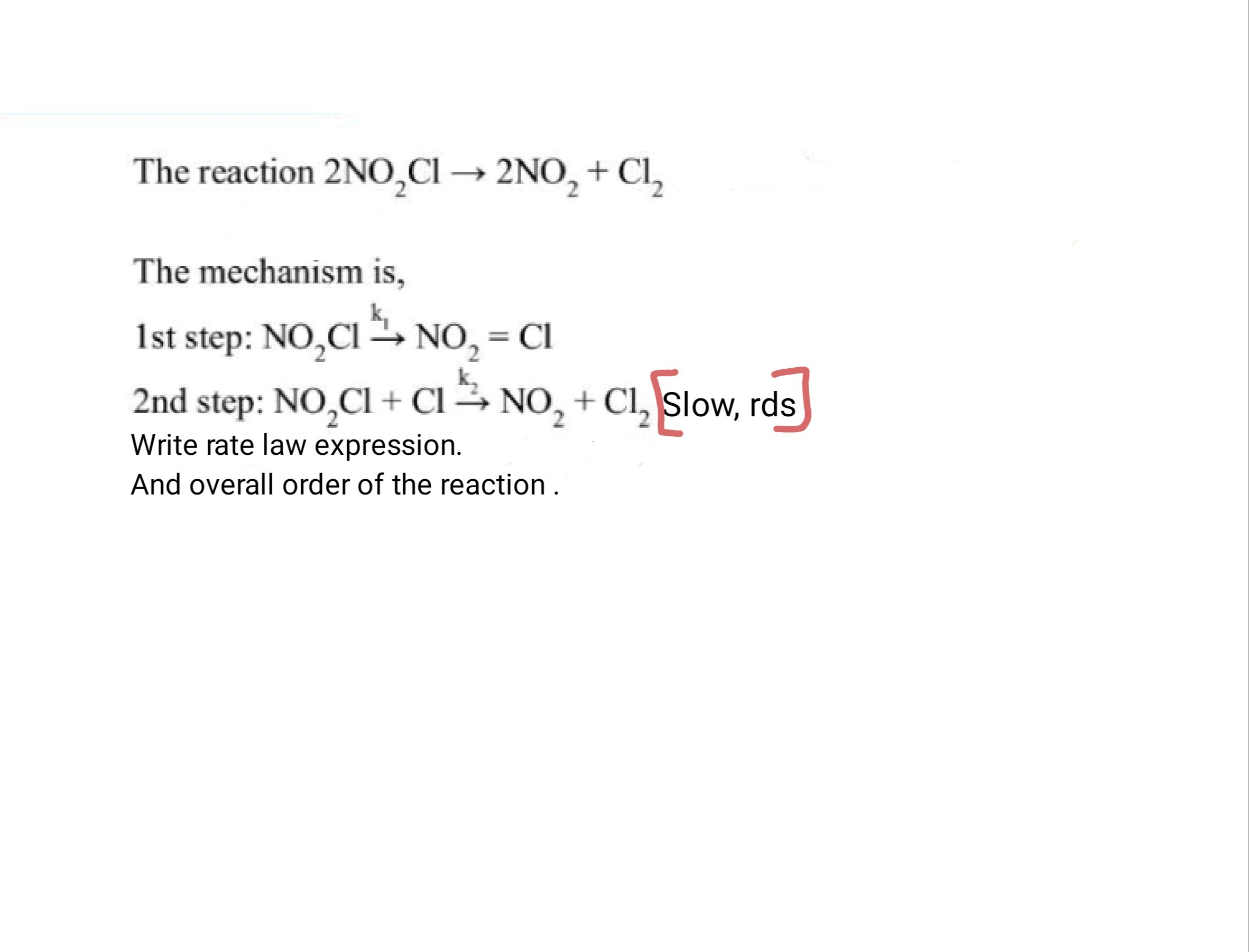
The reaction 2NO2CI→2NO2+Cl2
The mechanism is, 1ststep:NO2Clk1NO2=Cl 2ndstep:NO2Cl+Clk2NO2+Cl2 Slow, rds
Write rate law expression. And overall order of the reaction .
Rate = k[NO_2Cl], Order = 1
Solution
The rate law of a reaction mechanism is determined by the slowest step, which is the rate-determining step (rds). In the given mechanism, the second step is the slow step.
The mechanism is: 1st step: NO2Clk1NO2+Cl (Fast) 2nd step: NO2Cl+Clk2NO2+Cl2 (Slow, rds)
The rate law for the slow step is: Rate =k2[NO2Cl][Cl]
Here, Cl is an intermediate species, as it is produced in the first step and consumed in the second step. The rate law expression should be written in terms of the concentrations of the reactants of the overall reaction (NO2Cl). We need to express the concentration of the intermediate [Cl] in terms of the reactant concentration [NO2Cl].
Since the first step is fast and the second step is slow, the concentration of the intermediate [Cl] can be related to the reactant concentration. A common approach when a fast irreversible step produces an intermediate that is consumed in a subsequent slow step is to use the steady-state approximation for the intermediate. The steady-state approximation assumes that the net rate of change of the concentration of the intermediate is approximately zero.
Rate of formation of [Cl] in step 1 =k1[NO2Cl] Rate of consumption of [Cl] in step 2 =k2[NO2Cl][Cl]
According to the steady-state approximation: dtd[Cl]=Rate of formation−Rate of consumption=0 k1[NO2Cl]−k2[NO2Cl][Cl]=0
Assuming [NO2Cl]=0, we can divide by [NO2Cl]: k1=k2[Cl]
Solving for [Cl]: [Cl]=k2k1
Now substitute this expression for [Cl] into the rate law for the slow step: Rate =k2[NO2Cl][Cl] Rate =k2[NO2Cl](k2k1) Rate =k1[NO2Cl]
Let k=k1. The rate law expression is: Rate =k[NO2Cl]
The rate law shows that the rate of the reaction is directly proportional to the concentration of NO2Cl raised to the power of 1. The order of the reaction with respect to NO2Cl is 1. The overall order of the reaction is the sum of the exponents of the concentration terms in the rate law. In this case, the overall order is 1.
Rate law expression: Rate =k[NO2Cl] Overall order of the reaction: 1
