Question
Question: The ratio of the value of any colligative property for BaCl₂ solution and urea solution under simila...
The ratio of the value of any colligative property for BaCl₂ solution and urea solution under similar condition is
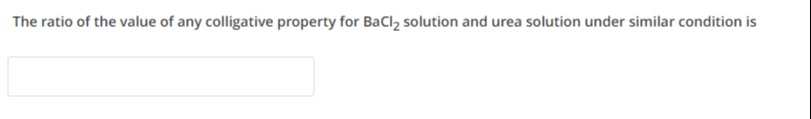
Answer
3
Explanation
Solution
Colligative properties depend on the number of solute particles.
For urea (non-electrolyte):
iurea=1
For BaCl2 (strong electrolyte):
BaCl2(s)→Ba2+(aq)+2Cl−(aq)
iBaCl2=3
Ratio = iureaiBaCl2=13=3
