Question
Question: The ratio of packing density in fcc, bcc and cubic structure is respectively ______. (A) 1 : 0.92 ...
The ratio of packing density in fcc, bcc and cubic structure is respectively ______.
(A) 1 : 0.92 : 0.70
(B) 0.70 : 0.92 : 1
(C) 1 : 0.70 : 0.92
(D) 0.92 : 0.70 : 1
Solution
Draw the diagram of a simple cubic, FCC and BCC unit cells. Each side of a simple cubic cell has length which equals two radii of two adjacent atoms. Volume of a sphere is equal to 34πr3.
Complete step by step answer:
For a simple cubic cell,
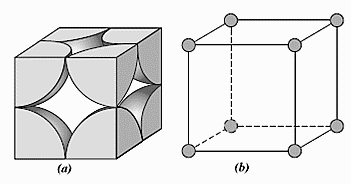
Length of each side of cubic cell, a=2r
Volume of one atom having radius (r) = 34πr3
There are 8 atoms occupying the corners of the cube. Each corner contains only 81th of an atom because one atom is surrounded by eight atoms. So, the total number of atoms present in a simple cubic cell =81×8=1.
Total volume of a unit cell =(2r)3=8r3
If an atom occupies the entire volume then, the volume occupied will be 100%. Now, the cubic cell contains only one atom and one atom can occupy only 34πr3 of volume. So, packing efficiency is 8r334πr3×100=52.4
Similarly, let us calculate for BCC unit cell,
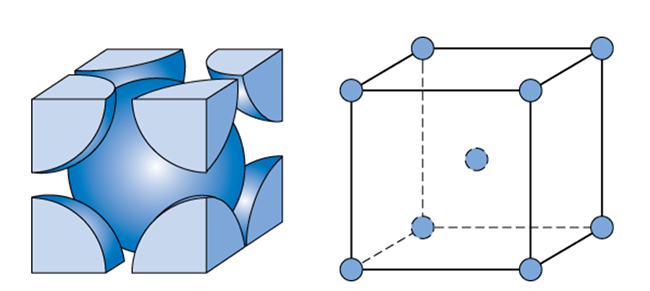
One atom is present in the centre of the unit cell and there are eight atoms present on the corners. Each corner contributes 81th of an atom because one atom is surrounded by eight atoms. So, the total number of atoms present in a simple cubic cell =(81×8)+1=2.
Volume occupied by two atoms in the BCC unit cell =2×34πr3=38πr3
In a BCC unit cell, consider AD as the diagonal of the cube and AB as the diagonal of a side of a cube. Consider a right-angled triangle ABC, where (length of the side of the cube). Applying Pythagoras theorem to △ABC, (AB)2=(AC)2+(BC)2=2a2.
Similarly, consider a right-angled triangle △ABD, where , (AB)2=2a2
(AD)2=(AB)2+(BD)2=2a2+a2=3a2AD=3a
But we know, the length of diagonal AD is 4r due to the presence of two radii of corner atoms and one diameter of central atom. So, equating the two values,
AD=3a=4r
∴a=34r
The total volume of cube =a3=3364r3
Therefore, packing efficiency of BCC unit cell is 38πr3×64r333×100=68.09
Similarly, let’s calculate for FCC cells.
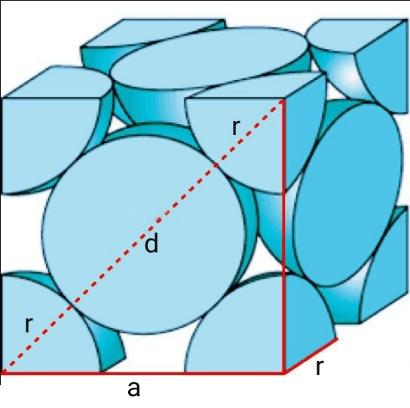
The FCC cell contains 81th atom at the 8 corners and 21th atom at the six faces.
Therefore, total atoms in a FCC cell =81×8+21×6=4.
Consider a right-angled triangle △ABC on the face of a FCC cell, where AC=BC=a and AB=4r. Similarly, (AB)2=(AC)2+(BC)2=a2+a2=2a2=16r2
Therefore, a=8r
Volume of the FCC unit cell =a3=88r3
Actual volume occupied by a cube of FCC =4×34πr3=316πr3
Hence, packing efficiency of FCC unit cell is 316πr3×88r31×100=74.0
Now taking the ratio of all the three we get, FCC : BCC : SC=1 : 0.92 : 0.70
So, the correct answer is “Option A”.
Note: Systematically calculate the volume occupied by atoms in each of the unit cells. Empty space or void in each cell can be calculated as well by just subtracting packing efficiency by a factor of 100.
