Question
Question: The ratio of magnetic dipole moment to angular momentum in hydrogen like atom is :- \[\begin{alig...
The ratio of magnetic dipole moment to angular momentum in hydrogen like atom is :-
& a)\dfrac{e}{m} \\\ & b)\dfrac{e}{2m} \\\ & c)\dfrac{e}{3m} \\\ & d)\dfrac{2e}{m} \\\ \end{aligned}$$Solution
When electron revolves in the circular orbit then that orbit will behave like a current carrying loop due to motion of electron and this current carrying loop has poles like magnet so it behaves like magnetic dipole. Then we use the general relation for magnetic moment and angular momentum for finding the ratio.
Complete answer:
An electron is revolving in an orbit about the nucleus of a hydrogen-like atom which behaves like a current loop and produces a magnetic field. So this current carrying loop behaves like magnetic dipole because its properties are matching with the properties of a magnet.
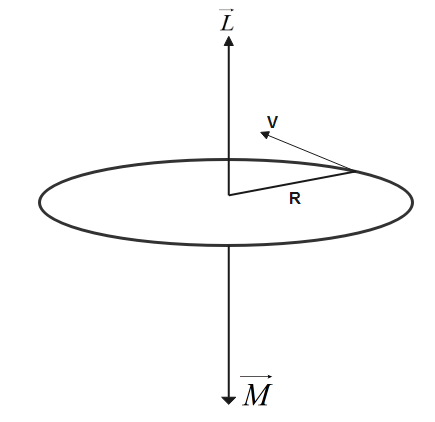
Let us assume the mass of electron revolving is m.
Let us assume the charge of a revolving electron is e.
An electron of mass m and charge is moving with speed v in a circular orbit of radius R. Due to motion of electrons in the loop it behaves like a current carrying loop.
Now we calculate the current I produced in the loop in time period of T is represented by formula.
According to formula,
i=TeEquation 1
Where T is the time period of revolution.
So Time(T)=SpeedDistance
⇒T=v2πR
Put the value of time period T in equation 1 is given by the equation -
i=2πRev(Equation2)
Let us assume Magnetic moment due to current carrying loop is represented by M
so formula for magnetic moment will be-
M=iA
Where A be the area of orbit of radius r.
So put the value of current i from equation 2 and area of loop A we get,
