Question
Question: The rate of esterification of acetic acid with I.Methyl alcohol II.Ethyl alcohol III.Isopropyl...
The rate of esterification of acetic acid with
I.Methyl alcohol
II.Ethyl alcohol
III.Isopropyl alcohol
IV.Tert-butyl alcohol
A.I > II > III > IV
B.IV > III > II > I
C.II > I > IV > III
D.III > IV > I > II
Solution
When carboxylic acids are heated with alcohols in the presence of concentrated sulphuric acid or dry hydrochloric acid gas, esters are formed and the process is known as esterification.
The rate of esterification is affected by steric hindrance of the alkyl groups. When the size and the number of the substituents around the carboxylic group or the hydroxyl group in alcohols increases, the rate of esterification slows down.
Complete step by step answer:
The esterification reaction is reversible in nature. A general form of the esterification reaction is shown below.
AcidRCOOH + AlcoholHOR’⇆conc.H2SO4EsterRCOOR’ + H2O
The esterification reaction involves the cleavage of the oxygen – hydrogen bond of alcohols and the acyl – oxygen bond of the carboxylic acids. The formation of tetrahedral intermediate during esterification is confirmed by the observation that the esterification rate is sensitive to steric hindrance.
Now, methyl alcohol or methanol is the simplest alcohol. Its structure is
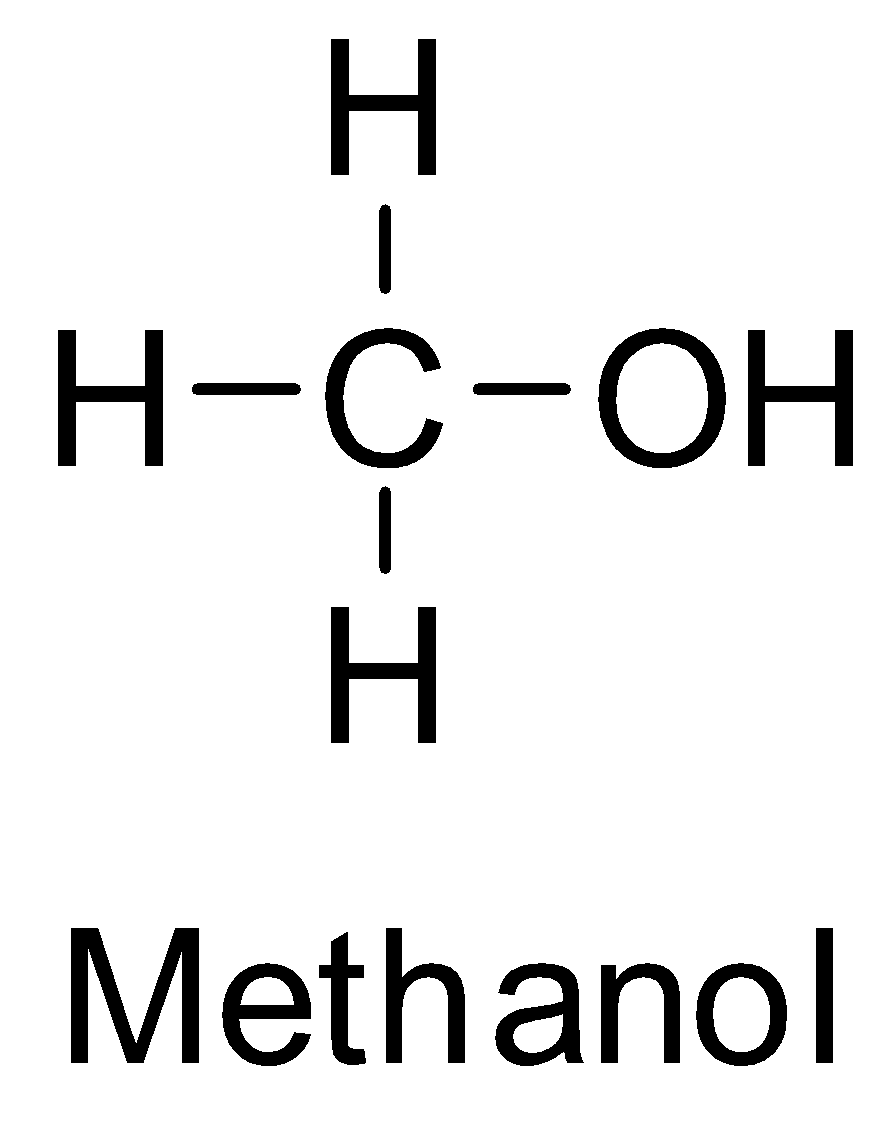
Thus, it has no steric hindrance and the rate of esterification is the fastest here.
Ethyl alcohol or ethanol is a primary alcohol and its structure is:
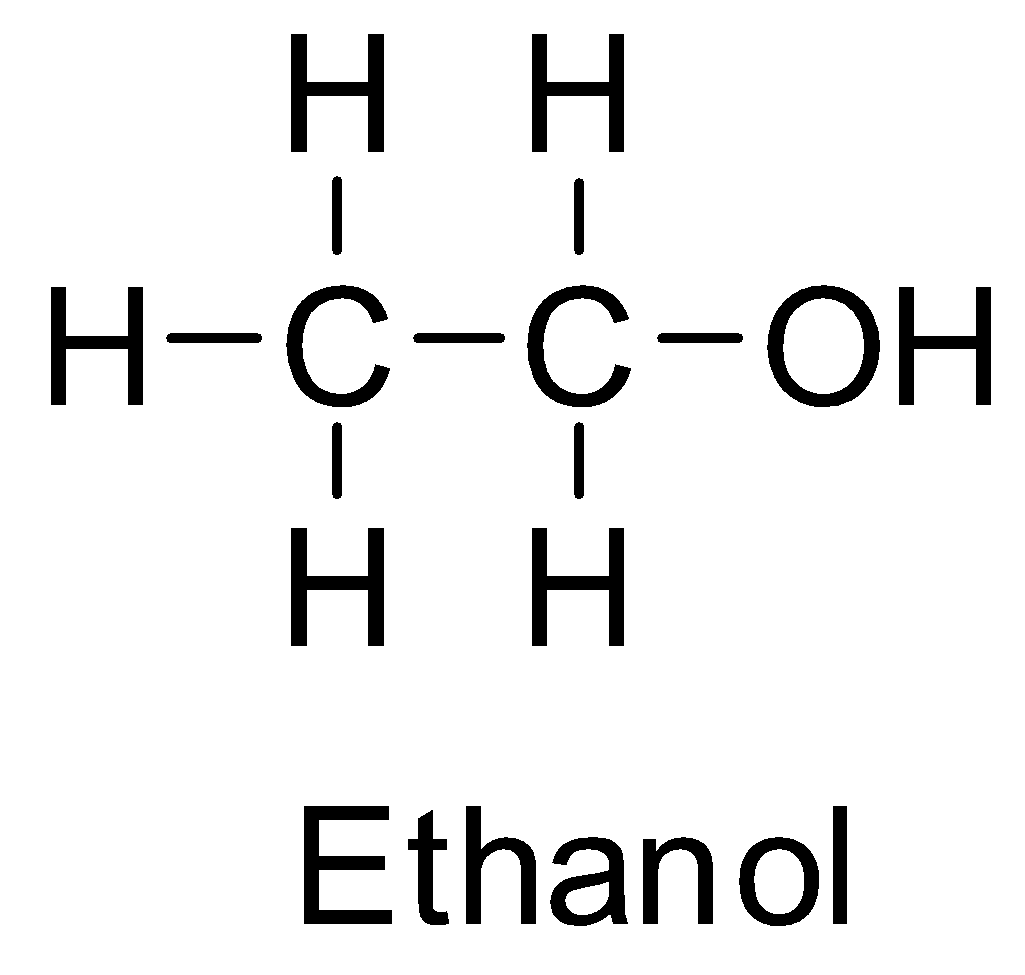
Isopropyl alcohol is a secondary alcohol and has the structure:
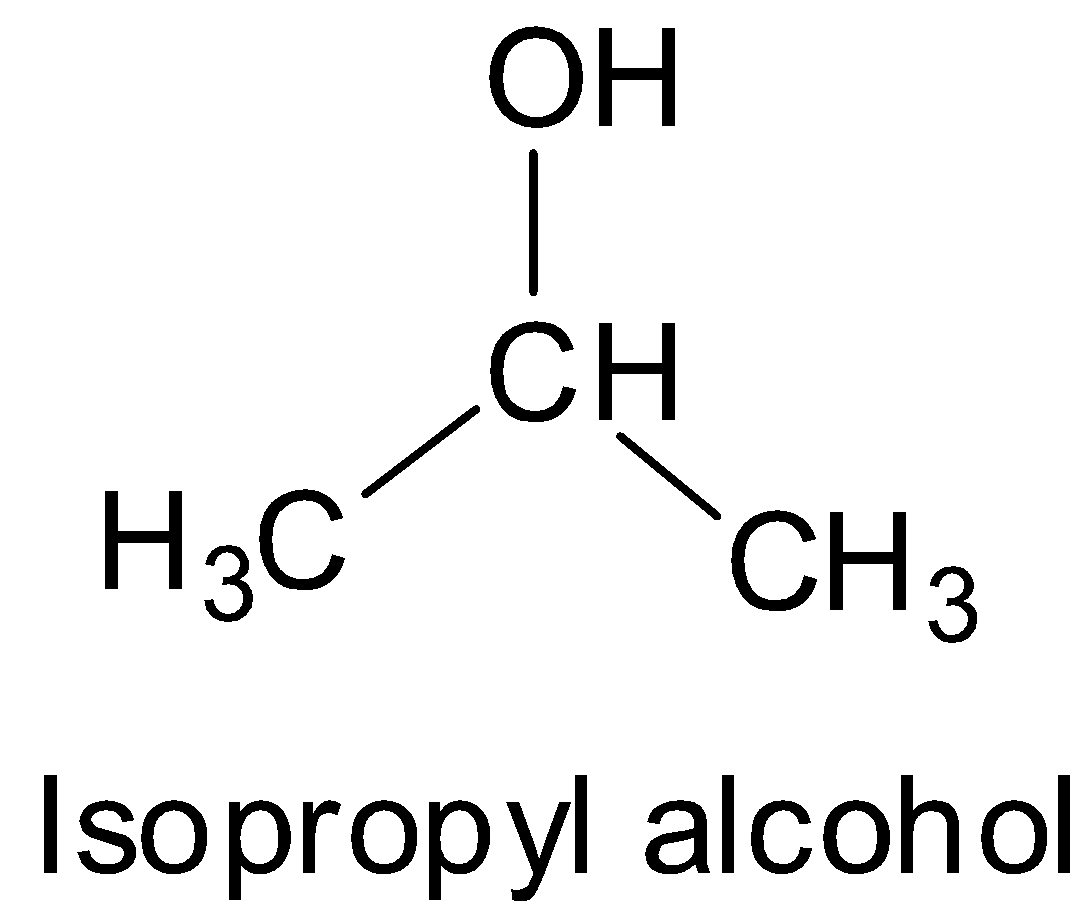
Tert-butyl alcohol is a tertiary alcohol and has the structure:
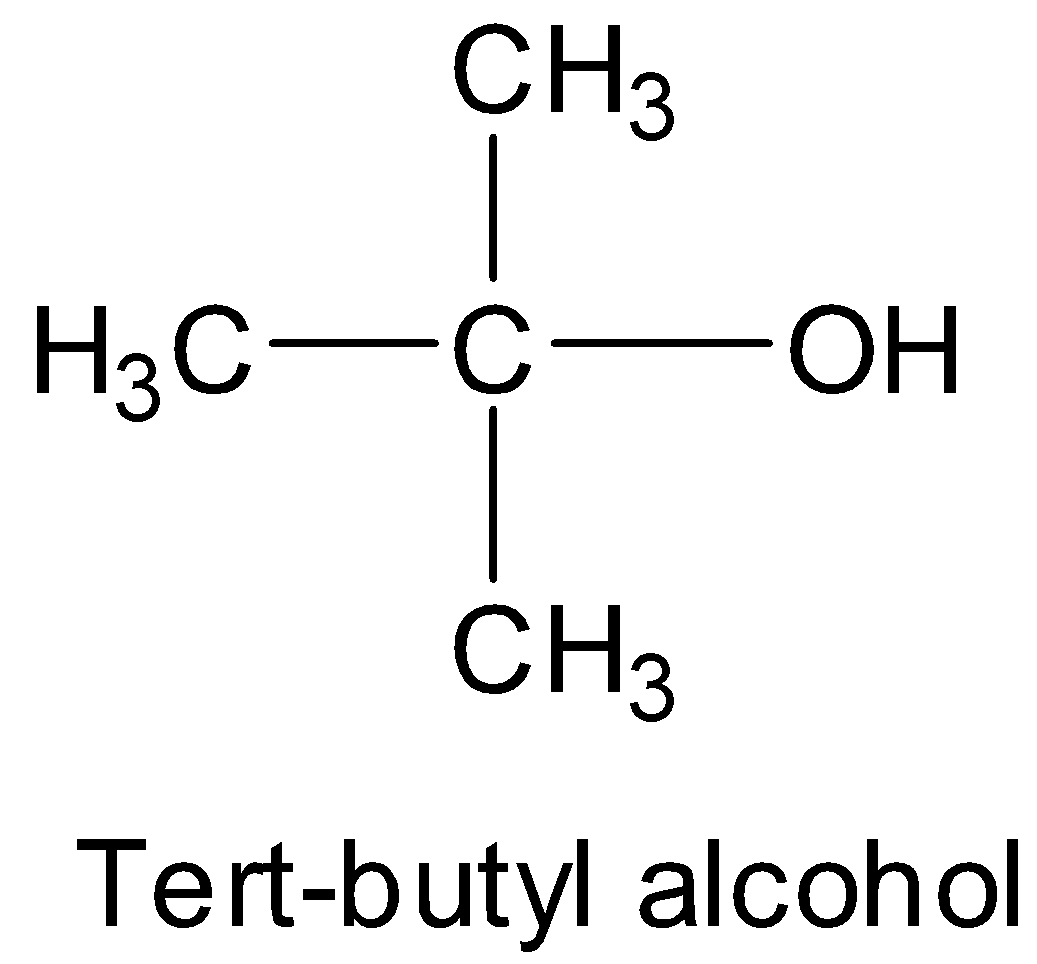
Thus, the bulkiness of the substituents around the hydroxyl groups increases from primary to secondary to tertiary. Hence, the order of reactivity is I > II > III > IV
Thus the correct option is A.
Note:
The esterification of carboxylic acids with alcohols is a nucleophilic acyl substitution reaction and involves three steps. The first step is the protonation of the carboxyl group to form protonated carboxylic acid.
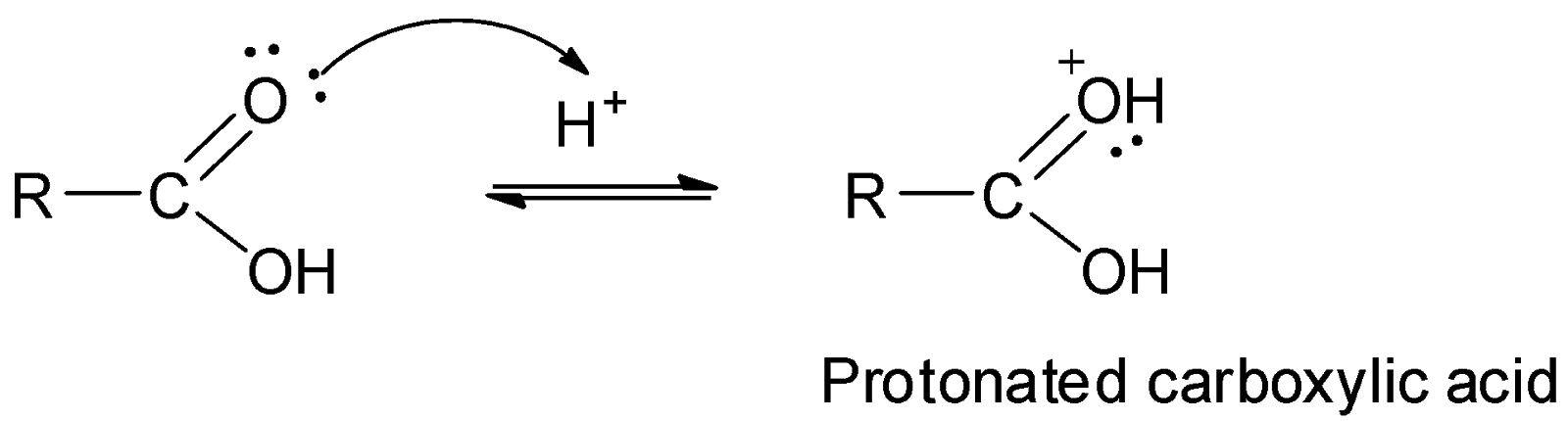
The second step involves the nucleophilic attack of the alcohol molecule on the carbonyl carbon to form a tetrahedral intermediate.
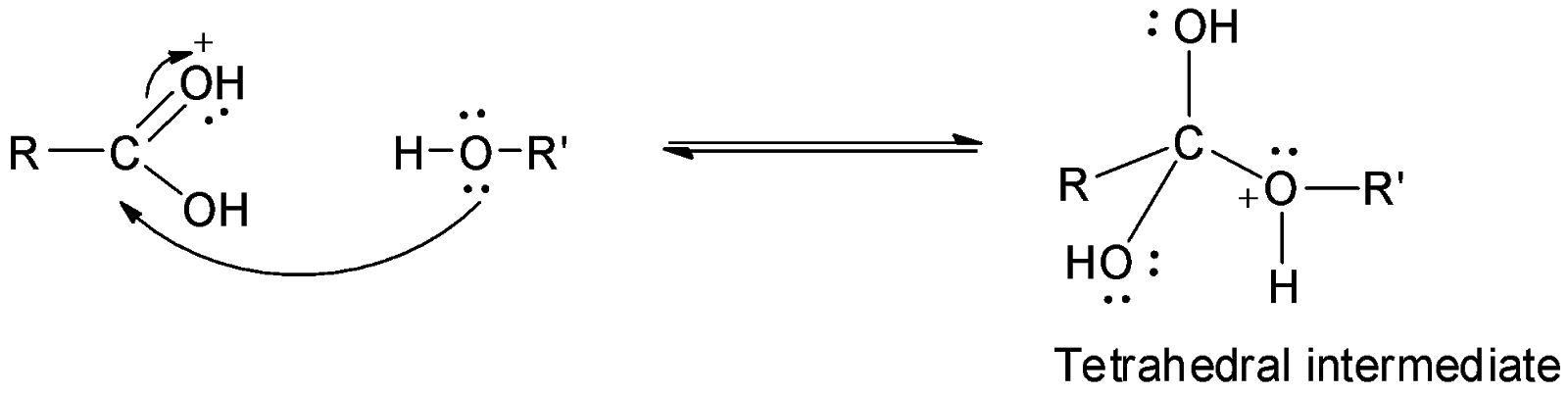
The third step is the loss of a molecule of water and a proton to form a protonated ester which finally loses a molecule to give the ester.
