Question
Question: The rate of elimination (using EtONa) is in the order:  is in the order:
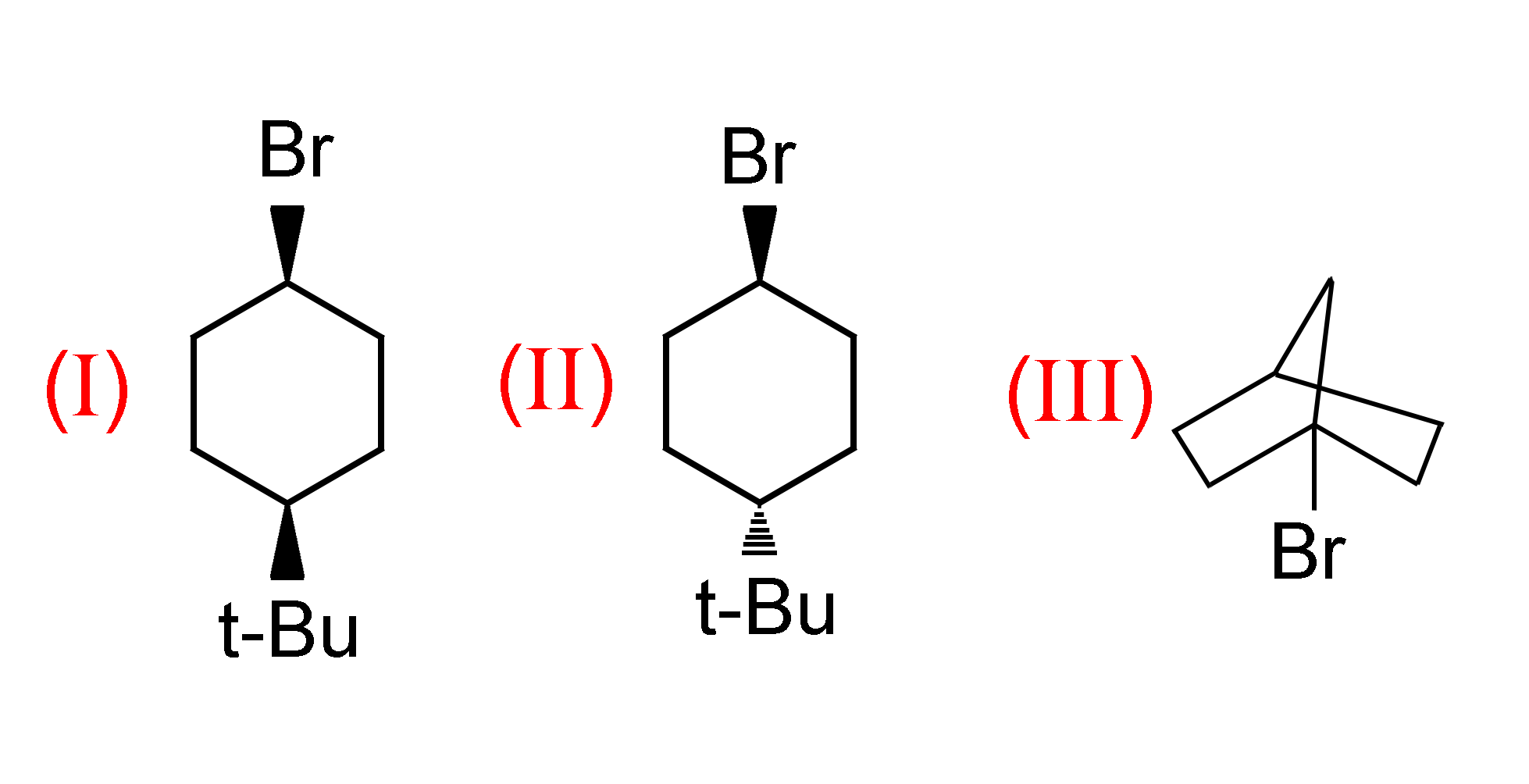
a.) I>II>III
b.) I>III>II
c.) II>I>III
d.) III>I>II
Solution
The C−Br bond will be more strained in the I compound and will be less strained in the II compound. In the case of the III compound the C−Br bond is present in a compact structure, so breaking the C−Br bond will be the hardest to break. So the order of elimination is I>II>III
Complete step by step answer:
The reaction of all the above compounds with sodium ethoxide (C2H5ONa) are examples of elimination reactions where Br is eliminated by the action of sodium ethoxide.
You may have noticed solid black lines and the line containing dashes in compounds I and II . These lines are used to represent the 3-dimensional structure of any compound, the solid black lines represent that the bond is in a direction away from the viewer and the dashed line which are called wedges represent that the direction of the bond is towards the viewer.
Now let’s consider the reaction of the I compound.
In this reaction Br is to be removed from the benzene ring. Br and t−Bu (Tertiary butyl) groups are present on the same side (i.e. on the side away from the viewer represented by black solid lines). Now since both these groups are on the same side, the bond between the C atom and Br atom will be more strained and thus Br atoms will be removed easily.
Now for the reaction of II compound.
In this compound Br and t−Bu groups are not present on the same side (i.e. t−Bu is represented by a dashed line and Br is represented by a solid line), so the bond between the C atom and Br atom will be less strained and thus Br atoms will be more difficult to remove.
Now for the reaction of the III compound.
Here Br is surrounded by three carbon atoms and is a compact structure, so here we can easily conclude that removing Br will be relatively difficult.
So, the order of rate of elimination using sodium ethoxide on the given compounds is I>II>III
So, the correct answer is “Option A”.
Note: In the solution above we discussed how the presence of groups around the C−Br bond affects the removal of Br from the compounds, this effect is called the steric effect (Steric effects are nonbonding interactions that affect the shape or conformation and reactivity of ions and molecules).
