Question
Question: The rate constants for decomposition of acetaldehyde have been measured over the temperature range 7...
The rate constants for decomposition of acetaldehyde have been measured over the temperature range 700-1000 K.
The data has been analysed by plotting lnk vs T103 graph. The value of activation energy for the reaction is ______ kJ mol−1. (Nearest integer) (Given : R = 8.31 J K−1 mol−1)
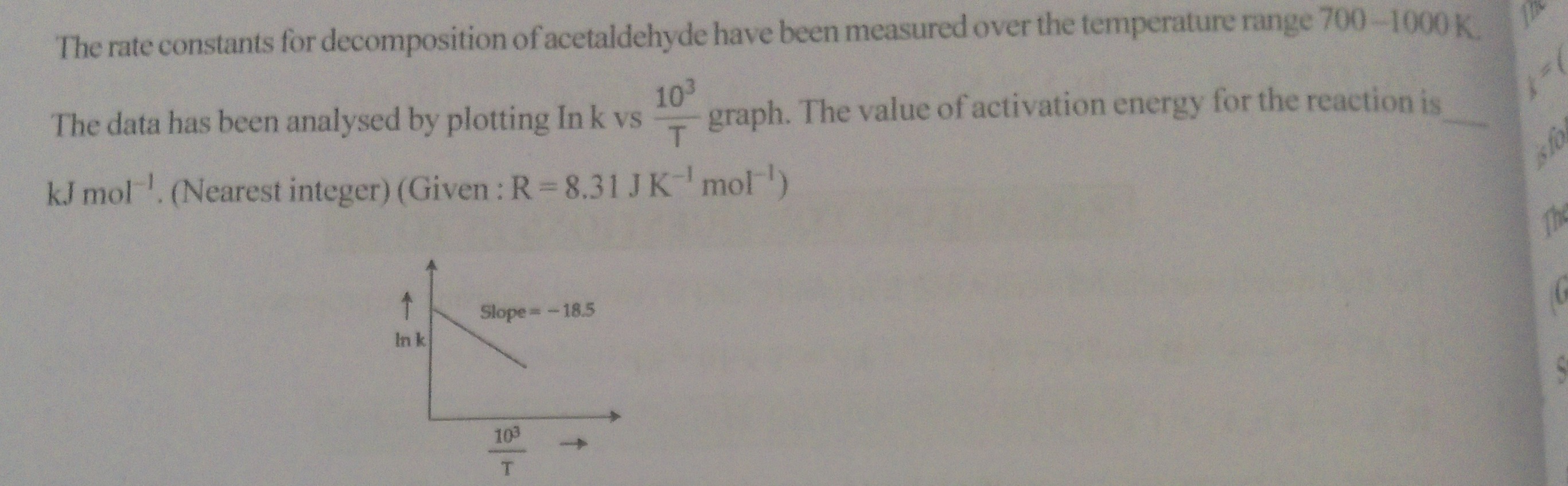
154 kJ mol−1
Solution
The Arrhenius equation in linear form is:
lnk=lnA−RTEa
When plotted as lnk vs. T103, note that:
T1=1031(T103)
Thus the equation becomes:
lnk=lnA−REa⋅1031(T103)
which is of the form lnk=constant+[−103REa](T103).
Given that the slope of this plot is −18.5, we have:
−103REa=−18.5⟹103REa=18.5
Solving for Ea:
Ea=18.5×103×R
With R=8.31JK−1mol−1,
Ea=18.5×103×8.31J mol−1≈153735J mol−1
Converting to kJ mol−1:
Ea≈153.7kJ mol−1≈154kJ mol−1(nearest integer).
