Question
Question: The radioactive decay series of $^{226}$Ra is as follows: The times indicated are half-lives, the u...
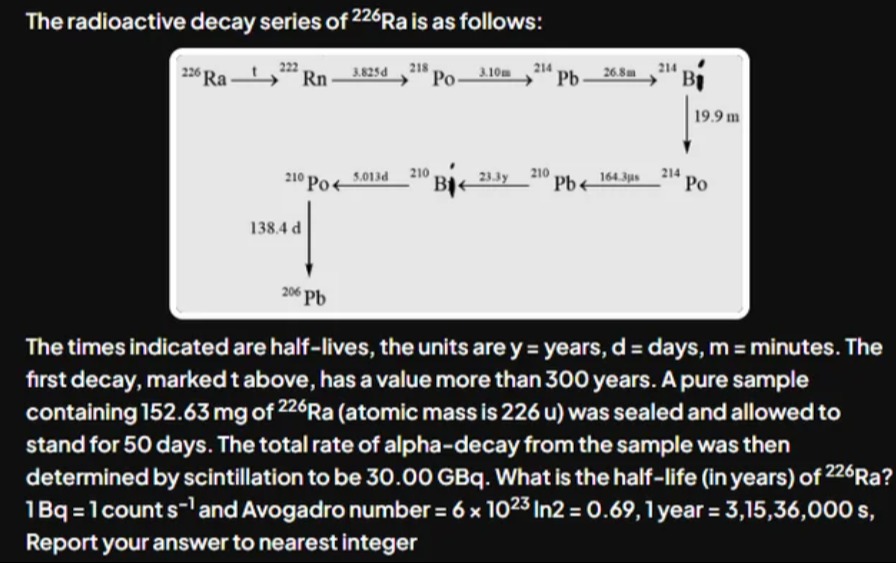
The radioactive decay series of 226Ra is as follows:
The times indicated are half-lives, the units are y = years, d = days, m = minutes. The first decay, marked t above, has a value more than 300 years. A pure sample containing 152.63 mg of 226Ra (atomic mass is 226 u) was sealed and allowed to stand for 50 days. The total rate of alpha-decay from the sample was then determined by scintillation to be 30.00 GBq. What is the half-life (in years) of 226Ra? 1Bq = 1 count s−1 and Avogadro number = 6 x 1023 In2 = 0.69, 1 year = 3,15,36,000 s, Report your answer to nearest integer
1187
Solution
We begin by noting that in the decay chain of ²²⁶Ra the following alpha‐emitters are present within 50 days:
- ²²⁶Ra itself (α emission)
- ²²²Rn (α emission; t₁/₂ = 3.825 d)
- ²¹⁸Po (α emission; t₁/₂ = 3.10 m)
- ²¹⁴Po (α emission; t₁/₂ = 19.9 m)
The later member ²¹⁰Po (α emitter) is produced via ²¹⁰Pb (t₁/₂ = 23.3 y) and so has not reached equilibrium in 50 d (its activity is only ≈0.4% of that from Ra). Thus, in a sealed sample allowed to stand 50 days, every ²²⁶Ra decay is “followed” by fast decays of its short‐lived daughters contributing 4 α decays.
Let A_Ra be the activity (in decays/s) of ²²⁶Ra. Then the measured total alpha count rate is
Atotal≈4ARa.We are told
Atotal=30.00 GBq=3.0×1010 s−1.Thus,
ARa=43.0×1010=7.5×109 s−1.Next, we find the number of ²²⁶Ra atoms in the sample. Given a mass of 152.63 mg:
Mass=0.15263 g,Molar mass=226 g/mol.Number of moles:
n=2260.15263≈6.76×10−4 mol.Using Avogadro’s number (6.0 × 10²³ mol⁻¹),
N=6.76×10−4×6.0×1023≈4.06×1020 atoms.The decay constant for Ra-226 is given by:
λ=NARa=4.06×10207.5×109≈1.85×10−11 s−1.Thus, the half-life is
T1/2=λln2=1.85×10−110.693≈3.74×1010 s.Convert seconds to years (1 year = 3.1536 × 10⁷ s):
T1/2≈3.1536×1073.74×1010≈1187 years.Minimal Explanation
- Only the short‐lived daughters ²²²Rn, ²¹⁸Po, ²¹⁴Po achieve equilibrium in 50 days. Total α count = 4× activity of ²²⁶Ra.
- ²²⁶Ra atoms calculated from 152.63 mg sample.
- Use A = λN and T₁/₂ = 0.693/λ to get T₁/₂ ≈ 1187 years.
Final Answer
Half-life of ²²⁶Ra ≈ 1187 years.
