Question
Question: The radii of the two columns in a \(U\) tube are \({r_1}\) and \({r_2}\), when a liquid of density (...
The radii of the two columns in a U tube are r1 and r2, when a liquid of density (angle of contact is 0o) is filled in it, the level difference of the liquid in the two arms in h. The surface tension of the liquid is: (g=acceleration due to gravity).
(A) 2(r2−r1)ρghr1r2
(B) 2r2r1ρgh(r2−r1)
(C) ρghr2r12(r1−r2)
(D) ρgh2(r1−r2)
Solution
Surface tension is nothing but the ability of liquid surfaces to shrink into minimum surface area. This property of a liquid allows it to block an external force as its molecules have cohesive nature.
Formula used:
The rise of liquid in the capillary tube can be calculated using the formula h=rρg2T.
Complete step by step answer:
The cohesive nature of the liquid molecules is responsible for resisting an external force and shrinking into the minimum surface possible. This phenomenon is called as surface tension.
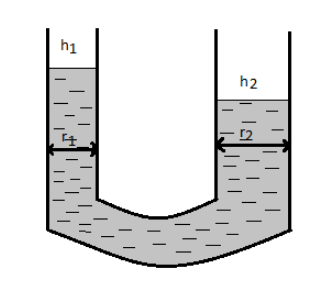
Let h1 be the height in the tube having radius r1.
So, we have h1=r1ρg2T−−−−−(1)
Similarly, let h2 be the height in the tube having radius r2.
So, we have h2=r2ρg2T−−−−−(2)
The level difference of liquid in the two arms can be given by
h=h1−h2
After substituting values from equations (1) and (2), we get
After rearranging the terms, we get T=2(r2−r1)hρgr1r2
Thus, we can conclude that the surface tension of the liquid is 2(r2−r1)ρghr1r2.
So, option A is the correct answer.
Note: In this example, the height h was mentioned, so we used the formula h=rρg2T. For other examples, force and length can also be used to find out the surface tension by using the formula, T=21×LFwhere T is the surface tension, F is the force, and L is the length.
