Question
Question: The radical given below is aromatic, because it has 
A. 7 p-orbitals and 6 unpaired electrons
B. 7 p- orbitals and 7 unpaired electrons
C. 6 p-orbitals and 7 unpaired electrons
D. 6 p-orbitals and 1 unpaired electron
Solution
Aromaticity is a chemical property of organic compounds, aromatic compounds follow Huckel’s rule. They have a high degree of stability. They show electrophilic substitution reactions. There is a diamagnetic current in these compounds.
Complete Step by step answer: aromatic compounds have high degree of stability due to filled bonding molecular orbital.
They follow huckel’s rule of aromaticity-
Huckel’s rule states that, for a compound to be aromatic, the compound should be planar, cyclic and should have (4n+2) pi electrons that should be in continuous delocalization or an uninterrupted cyclic pi electron cloud should be there. Here n= 0,1,2……
So if we put n=0 ,we get 2 pi electrons
If we put n=1, we get 6 pi electrons
If we put n=2, we get 10 pi electrons and so on
For example benzene, we all know are aware of the structure of benzene. It is planar, it is cyclic and it has 6 Pi electrons
In the above case, it is aromatic, because it is planar, cyclic and has 6 pi electrons which are in continuous delocalization. And hence satisfying the condition of Huckle’s rule of aromaticity.
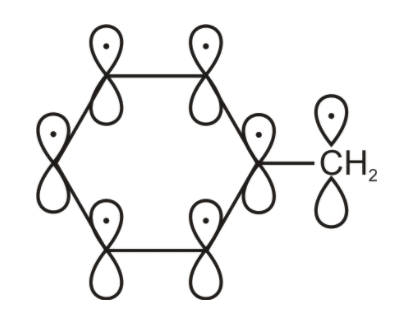
But the free radical also has 1 unpaired electron and 1 p orbital. Here the free radical unpaired electron is also involved in the delocalization. So total we have 7 unpaired electrons 7 p- orbitals.
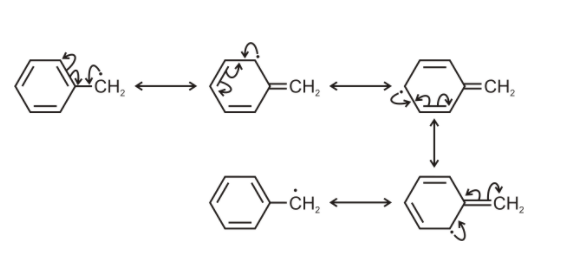
Hence the correct option will be B. total 7 unpaired electrons and 7 p- orbitals.
Note: Molecules which are cyclic, planar, and have 4n pi electrons which are in conjugation are called as anti-aromatic. For example: cyclobuta-1,3-diene. The compounds which do not follow huckel’s rule for aromaticity and anti-aromaticity are non-aromatic. For example: cyclooctatetraene.
